
Title: The micro-organisms of the soil
Author: Edward J. Russell
Rothamsted Experimental Station
Release date: August 3, 2022 [eBook #68670]
Most recently updated: October 18, 2024
Language: English
Original publication: United Kingdom: Longmans, Green and Co
Credits: Charlene Taylor, Harry Lamé and the Online Distributed Proofreading Team at https://www.pgdp.net (This file was produced from images generously made available by The Internet Archive/Canadian Libraries)
Please see the Transcriber’s Notes at the end of this text.
The cover image has been created for this text and is in the public domain.

THE ROTHAMSTED MONOGRAPHS ON
AGRICULTURAL SCIENCE
EDITED BY
Sir E. J. RUSSELL, D.Sc. (Lond.), F.R.S.
THE MICRO-ORGANISMS OF THE SOIL
THE ROTHAMSTED MONOGRAPHS ON
AGRICULTURAL SCIENCE.
Edited by Sir E. JOHN RUSSELL, D.Sc., F.R.S.
During the past ten years there have been marked developments in knowledge of the relations between the soil and the growing plant. The subject involves physical, biological, and chemical considerations, and its ramifications are now so wide that they cannot be satisfactorily dealt with in detail in any one book. These monographs collectively cover the whole ground. In “Soil Conditions and Plant Growth” the general outlines are presented: in the monographs the various divisions are fully and critically dealt with by the Heads of the Departments concerned at Rothamsted. A homogeneous treatment is thus secured that will, it is hoped, much facilitate the use of the series.
SOIL CONDITIONS AND PLANT GROWTH, Fourth Edition.
By Sir E. John Russell, F.R.S. 16s. net.
The following volumes are in preparation:—
| MANURING OF GRASS-LANDS FOR HAY | By Winifred E. Brenchley, D.Sc., F.Z.S. |
| THE MICRO-ORGANISMS OF THE SOIL | By Sir E. John Russell, F.R.S., and Members of the Biological Staff of the Rothamsted Experimental Station. |
| SOIL PHYSICS | By B. A. Keen, B.Sc. |
| SOIL PROTOZOA | By D. W. Cutler, M.A., and L. M. Crump, M.Sc. |
| SOIL BACTERIA | By H. G. Thornton, M.A. |
| SOIL FUNGI AND ALGÆ | By W. B. Brierley, S. T. Jewson, B.Sc., and B. M. Roach (Bristol), D.Sc. |
| CHEMICAL CHANGES IN THE SOIL | By H. J. Page, B.Sc. |
LONGMANS, GREEN AND CO.,
LONDON, NEW YORK, TORONTO, BOMBAY, CALCUTTA, AND MADRAS.
BY
Sir E. JOHN RUSSELL, F.R.S.
AND
MEMBERS OF THE BIOLOGICAL STAFF OF THE
ROTHAMSTED EXPERIMENTAL STATION
WITH DIAGRAMS
LONGMANS,
GREEN AND CO.
39 PATERNOSTER ROW, LONDON, E.C. 4
NEW YORK, TORONTO
BOMBAY, CALCUTTA and MADRAS
1923
Made in Great Britain
[v]
The purpose of this volume is to give the broad outlines of our present knowledge of the relationships of the population of living organisms in the soil to one another and to the surface vegetation. It is shown that there is a close relationship with vegetation, the soil population being dependent almost entirely on the growing plant for energy material, while the plant is equally dependent on the activities of the soil population for removing the residues of previous generations of plants and for the continued production in the soil of simple materials, such as nitrates, which are necessary to its growth. It is also shown, however, that the soil population takes toll of the plant nutrients and that some of its members may directly injure the growing plant.
The soil population is so complex that it manifestly cannot be dealt with as a whole in any detail by any one person, and at the same time it plays so important a part in the soil economy that it must be seriously studied. Team work therefore becomes indispensable, and fortunately this has been rendered possible at Rothamsted.
Each group of organisms is here dealt with by the person primarily responsible for that particular section of the work. The plan of the book has been carefully discussed by all the authors, and the subject matter has already been presented in a course of lectures given at University College, London, under the auspices of the[vi] Botanical Board of Studies of the London University. The interest shown in these lectures leads us to hope that the subject may appeal to a wider public, and above all to some of the younger investigators in biological science. They will find it bristling with big scientific problems, and those who pursue it have the satisfaction, which increases as the years pass by, of knowing that their work is not only of interest to themselves, but of great importance in ministering to the intellectual and material needs of the whole community.
[vii]
| CHAP. | PAGE | |
|---|---|---|
| I. | Development of the Idea of a Soil Population | 1 |
| Sir E. John Russell, F.R.S., Director. | ||
| II. | Occurrence of Bacteria in Soil—Activities connected with the Acquirement of Energy | 20 |
| H. G. Thornton, B.A., Head of the Department of Bacteriology. | ||
| III. | Conditions affecting Bacterial Activities in the Soil—Activities connected with the Intake of Protein Building Materials | 39 |
| H. G. Thornton, B.A., Head of the Department of Bacteriology. | ||
| IV. | Protozoa of the Soil, I. | 66 |
| D. W. Cutler, M.A., Head of the Department of Protozoology. | ||
| V. | Protozoa of the Soil, II. | 77 |
| D. W. Cutler, M.A., Head of the Department of Protozoology. | ||
| VI. | Soil Algæ | 99 |
| B. Muriel Bristol, D.Sc., Algologist. | ||
| VII. | Soil Fungi—The Occurrence of Fungi in the Soil | 118 |
| W. B. Brierley, D.Sc., Head of the Department of Mycology. | ||
| VIII. | Soil Fungi—The Life of Fungi in the Soil | 131 |
| W. B. Brierley, D.Sc., Head of the Department of Mycology. | ||
| IX. | The Invertebrate Fauna of the Soil (other than Protozoa) | 147 |
| A. D. Imms, D.Sc., Head of the Department of Entomology. | ||
| X. | The Chemical Activities of the Soil Population and their Relation to the Growing Plant | 164 |
| Sir E. John Russell, F.R.S., Director. | ||
| Index | 181 |
[1]
From the earliest times agriculturists have been familiar with the idea that decomposition of vegetable and animal matter takes place in the soil, and that the process is intimately connected with soil fertility.
By the middle of the nineteenth century three different ways were known in which the decomposition occurred. One had been since early times specially associated with soil fertility, in that it gave rise to humus, the black sticky substance in farmyard manure or in soil—which was supposed up to 1840 to be the special food of plants. No good account of the process or of the conditions in which it occurred is, however, given by the older writers.
A second resulted in the formation of nitrates. This process became known as nitrification: it was described by Georgius Agricola (1494-1555) in his book “De Re Metallica,” and it was of great importance in the seventeenth and eighteenth centuries, because it was used for the manufacture of gunpowder in the great wars of that period. The conditions for the making of successful nitre beds were so thoroughly investigated that little fresh knowledge was added to that of 1770[A] until quite recently. This process, however, was not usually associated with soil fertility, although both Glauber (1656) and Mayow (1674) had insisted on the connection.
[A] See the remarkable collection of papers entitled “Instructions sur l’établissement des nitrières,” publié par les Régisseurs-généraux des Poudres et Salpêtre. Paris, 1777.
[2]
A third type of decomposition was brought into prominence by Liebig in 1840.[7] [B] Reviewing the decomposition of organic matter in the light of the newer chemistry, he concluded that the process was a slow chemical oxidation, to which he gave the name “Eremacausis.” He recognised that humus was formed, but he regarded it only as an intermediate product, and emphatically denied its importance in soil fertility. The true fertility agents, in his view, were the final products—CO2, potassium and other alkaline salts, phosphates, silicates, etc. He went on to argue brilliantly that instead of applying farmyard or similar manures to the soil it was altogether quicker and better to apply these mineral compounds obtained from other sources than to wait for the slow process of liberation as the result of decomposition. For some reason, difficult to understand, he overlooked nitrification and the part that nitrates might play in soil fertility. Lawes and Gilbert[6] were much attracted by this new idea; they showed that it was incomplete because it took no account of the necessity for supplying nitrogen compounds to the crop. When ammonium salts were added to Liebig’s ash constituents the resulting mixture had almost as good a fertilising effect as farmyard manure. Lawes at once saw the enormous practical importance of this discovery, and set up a factory for the manufacture of artificial fertilisers. He did not, however, follow it up more closely on the scientific side.
Both Lawes and Gilbert were in constant touch with the idea of decomposition in the soil, and they attached so much importance to nitrogen compounds in plant nutrition that it is not easy to understand how they missed the connection with nitrification. But they did so, and like other English and German workers of the day, considered that plant roots assimilated their nitrogen as ammonia. For the first ten years of the history of Rothamsted only few experiments with nitrates were made, and not till thirty-five years had elapsed were they systematically studied.
[3]
It was by Boussingault[2] and in France that the connection between nitrification and soil fertility was first recognised. The news came to England, but it was not accepted, although Way, one of the most brilliant agricultural chemists of his time, showed that nitrates were formed in soils to which nitrogenous fertilisers were added, and that they were comparable in their fertiliser effects with ammonium salts.[12] “The French chemists,” he wrote in 1856, “are going further, several of them now advocating the view that it is in the form of nitric acid that plants make use of compounds of nitrogen. With this view I do not at present coincide, and it is sufficient here to admit that nitric acid in the form of nitrates has at least a very high value as manure.” Indeed, Kuhlmann went so far as to argue that the nitrates found in the soil were there reduced to ammonia before assimilation by plants could take place. The water-culture work of the plant physiologists of the ’sixties finally showed the correctness of the French view.
Even when the importance of nitrification was realised its mechanism was not understood: some thought it was chemical, some physical. Again the explanation came from France. Pasteur in 1862 had expressed the view that nitrification would probably be a biological action, since purely chemical oxidation of organic matter was of very limited occurrence. “Pénétrés de ces idées,” as Schloesing tells us, he and Müntz in a memorable investigation cleared up the whole problem, and in 1877 opened the way to a most fruitful field of research.[10] The formal description is given in his papers in the “Comptes Rendus,” but a more lively account is given in his lectures before the École d’application des Manufacteurs de l’état, which, though not printed, were collected and issued in script by his distinguished son, and a copy of this work is among the treasures of the Rothamsted Library.
He had been asked to study the purification of sewage, and he and Müntz showed that it was bound up with nitrification. The process was slow in starting, then it proceeded[4] rapidly. Why, they asked, was the delay? There should be none if the process were physical or chemical, and the fact that it occurred strongly suggested biological action. The process was stopped by chloroform vapour, but could be restarted after the removal of the vapour by the addition of a little fresh soil.
The importance of this work in connection with soil fertility was immediately realised by Warington, who had recently come to Rothamsted.[11] He quickly confirmed the result, and made the valuable discovery that two stages were involved—the conversion of ammonia to a nitrite by one organism, and of the nitrite to nitrate by another. He made long and persistent attempts to isolate the organisms from the soil, using the best technique of his time, but though he found many bacteria none of them could nitrify ammonium salts; yet the soil did it easily. For years he continued his efforts to find the nitrifying organism, but always failed. His health was not good, his life at Rothamsted was not happy owing to disagreements with Gilbert, and although his other research work was succeeding, this investigation on which he had set his heart was not coming out; bacterial technique was not yet sufficiently far advanced. Ten bitter, disappointing years passed, and the crown of disappointment came when Winogradsky, a young bacteriologist in Paris, changed the technique and succeeded at once in isolating both the nitrite and the nitrate-forming organisms.[13]
The numerous bacteria found by Warington in the soil suggested the presence of a soil population, and this idea was greatly strengthened by another line of investigation which was being followed up in France. Boussingault had shown that soils absorb oxygen and give out carbon dioxide; Schloesing extended this discovery, as also did Wollny. It was concluded that oxidation was the result of the activities of the soil organisms in decomposing the organic matter of the soil, and thus preparing the way for the nitrifying organisms.
A third important function of soil bacteria was revealed[5] by Berthelot.[1] It was known that considerable loss of nitrogen from the soil took place as the result of the conversion of nitrogen compounds into nitrates, which were subsequently washed out in the drainage water. It followed inevitably that the stock of nitrogen compounds in the soil must long ago have become exhausted had there been no addition of nitrogen compounds to the soil. Berthelot argued that there must be fixation of atmospheric nitrogen, and, following the prevailing trend of thought in France, he attributed it to bacteria. He confirmed the anticipation by exposing soil to air in such conditions that dust, rain, etc., were excluded, and he found an increase in the percentage of nitrogen.
Looking back over the work, it is difficult to understand the result. The fixation of nitrogen is a process that absorbs energy, and should have necessitated some source of energy, which apparently was not supplied. But in spite of this drawback the investigation was immediately fruitful in that it gave the key to another problem which had long puzzled agriculturists.
It had long been known that the growth of leguminous crops, unlike that of others, enriched the ground,[C] and Lawes and Gilbert had shown that this was due to an increase of soil nitrogen. But no explanation could be found till Hellriegel and Wilfarth solved the problem.[4] In studying the nitrogen nutrition of gramineous and leguminous crops, they discovered that the gramineous plants died in absence of nitrate, and in its presence made growth which increased regularly with nitrate supply; while leguminous plants sometimes died and sometimes flourished in absence of nitrate, and behaved equally erratically with increasing nitrate supply. When the plants flourished nodules were[6] invariably present on the roots, but not otherwise. They concluded, therefore, that the nitrogen nutrition of leguminous plants differed from that of the gramineæ, and depended on some factor which sometimes came into their experiments and sometimes did not, and, in any case, was associated with the nodule. Knowing that the nodules on the roots of leguminous plants contained bacteria-like bodies, and remembering Berthelot’s results, they explored the possibility of bacterial fixation. They sterilised the sand and found that peas invariably failed to develop nodules and died, but after adding a little garden soil nodules were found and vigorous growth was obtained.
[C] “Of the leguminous plants the bean best reinvigorates the ground ... because the plant is of loose growth and rots easily, wherefore the people of Macedonia and Thessaly turn over the ground when it is in flower” (i.e. dig it into the ground if the soil is poor). Theophrastus, “Enquiry into Plants,” bk. viii. 2, and bk. ix. I. This book is of profound interest to agriculturists and botanists. An excellent translation by Sir Arthur Hort is now available. (Loeb’s Classical Library.)
Chemical analysis showed considerable fixation of gaseous nitrogen, which Hellriegel associated with the nodule organism. This has proved to be correct, and the fixation of nitrogen by bacteria is now a well-recognised process, the conditions of which are being thoroughly worked out. Two types of organisms are known—those associated with leguminous plants, and those living in a free and independent state in the soil. Of the latter the Clostridium, isolated by Winogradsky, is anaerobic, and the Azotobacter of Beijerinck is aerobic. The essential conditions are that a source of energy must be supplied—usually given as sugar—that the medium must not be acid, and that sufficient phosphate must be present.
All this brilliant work had been accomplished in the short space of the ten years 1880 to 1890. The inspiration had in each instance come from France, and is traceable direct to Pasteur, although coming long after his own work on bacteriology. It is impossible for us now to realise the thrill of wonder and astonishment with which students, teachers, and writers of those days learned that the nutrition of plants, and therefore the growth of crops and the feeding of themselves, was largely the result of the activity of bacteria in the dark recesses of the soil. It is not surprising that the ideas were pushed somewhat too far, that the soil population was regarded as solely bacterial, and that important[7] chemical and physical changes were sometimes overlooked.
Gradually there came the inevitable reaction and a somewhat changed outlook. Continued examination showed the presence in soil of almost every kind of bacteria for which search was made. Some of them were almost certainly in the resting condition as spores, and the new generation of workers had an uneasy feeling that the case for the overwhelming importance of bacteria in the economy of the soil was not too well founded. It was shown that the decomposition of nitrogen compounds to form ammonia would take place without micro-organisms if, as presumably would happen, the plant enzymes continued to act after they got into the soil. Even the oxidation of ammonia to nitrate—the great stronghold of the biological school—was accomplished by chemical agents. The fixation of nitrogen in soil conditions was beyond the power of chemists to achieve, and here it was universally agreed that bacteria were the active agents. And finally, chemists were themselves bringing into prominence a set of bodies—the colloids—whose remarkable properties seemed indefinitely expansible, and were in addition sufficiently incomprehensible to the ordinary student to attain much of the magnificence of the unknown.
All the time, however, a faithful body of workers was busy exploring the ground already won, improving the technique, making counts of the numbers of bacteria in the soil, and trying to measure the amount of bacterial activity. Much of the value of this work was limited by the circumstance that the bacteria were regarded as more or less constant in numbers and activities, so that a single determination was supposed to characterise the position in a given soil.
This was the condition of the subject when it was seriously taken up at Rothamsted. Before turning to agriculture, the writer had been studying the mechanism of certain slow chemical oxidations, and one of his first experiments in agriculture was to examine the phenomena of oxidation in soil. The results accorded with the biological explanation[8] of Schloesing: when the soil was completely sterilised oxidation almost ceased. But the striking discovery was made, as the result of an accident to an autoclave, that partial sterilisation increased the rate of oxidation, and therefore presumably the bacterial activity. This remarkable phenomenon had, however, already been observed, and it had been shown that both bacterial numbers and soil fertility were increased thereby. A full investigation was started in 1907 by Dr. Hutchinson and the writer.[9] From the outset the phenomena were recognised as dynamic and not static, and the rates of change were always determined: thus the bacterial numbers, the nitrate and ammonia present were estimated after the several periods. Close study of the curves showed that the chemical and bacterial changes were sufficiently alike to justify the view that bacteria were in the main the causes of the production of ammonia and of nitrate; although non-biological chemical action was not excluded, there was no evidence that it played any great part. Thus the importance of micro-organisms in the soil was demonstrated.
The factor causing the increased bacterial numbers after partial sterilisation was studied by finding out what agents would, and what would not, allow the numbers to increase, e.g. it was found that the bacterial increases became possible when soil had been heated at 56° C., but not at 40° C. Again, it was shown that the high numbers in partially sterilised soils rose for a time even higher if a little fresh untreated soil were incorporated into the partially sterilised soil, but afterwards they fell considerably. Putting all the results together, it appeared that some biological cause was at work depressing the numbers of bacteria in normal soils, but not—or not so much—in the partially sterilised soils. Studied in detail, the data suggested protozoa as the agent keeping down bacterial numbers, and they were found in the untreated, but not in the treated, soils. The hypothesis was therefore put forward that bacteria are not the only members of the soil population, but that protozoa are also present[9] keeping them in check, and therefore adversely affecting the production of plant food.
This conclusion aroused considerable controversy. It was maintained that protozoa were not normal inhabitants of the soil, but only occasional visitants, and, in any case, they were only there as cysts; the soil conditions, it was urged, were not suitable to large organisms like protozoa. The objection was not to be treated lightly, but, on the other hand, the experiments seemed quite sound. As neither Dr. Hutchinson nor the writer were protozoologists, Dr. T. Goodey and (after he left) Mr. Kenneth R. Lewin were invited to try and find out, quite independently of the partial sterilisation investigation, whether protozoa are normal inhabitants of the soil, and if so, whether they are in a trophic condition, and what is their mode of life and their relation to soil bacteria. Had it turned out that protozoa had nothing to do with the matter, search would have been made for some other organism. Goodey showed that the ciliates were not particularly important; Lewin soon demonstrated the existence of trophic amœbæ and flagellates. Unfortunately he was killed in the war before he had got far with the work. After the Armistice, Mr. Cutler accepted charge of the work: he will himself relate in Chapters IV. and V. what he has done.
At first sight it might be thought comparatively easy to settle a question of this kind by examining soil under a microscope or by sterilising it and introducing successively bacteria and known types of protozoa. Unfortunately neither method is simple in practice. It is impossible to look into the soil with a microscope, and methods of teasing-out small pieces of soil on a slide under the high, or even the low power, give no information, because the particles of soil have the remarkable power of attracting and firmly retaining protozoa, and no doubt bacteria as well; indeed, for protozoa (which have been the more fully investigated) there seems to be something not unlike a saturation capacity (see Fig. 9, p. 78). Further, complete sterilisation of soil[10] cannot be effected without at the same time altering its chemical and physical properties, and changing it as a habitat for micro-organisms. Cutler has, however, overcome the difficulties and shown that the introduction of protozoa into soils sterilised and then reinfected with bacteria considerably reduces the numbers of these organisms.
The method adopted, therefore, is to take a census of population and of production. Counting methods are elaborated, and estimates as accurate as possible are made of the numbers of the various organisms in a natural field soil at stated intervals. Simultaneously, wherever possible some measure is taken of the work done. The details of the census are finally arranged in consultation with the Statistical Department, to ensure that the data shall possess adequate statistical value. From the results it is possible to adduce information of great value as to the life of the population, the influence of external conditions, etc.
The most important investigation of this kind carried out at Rothamsted was organised by Mr. Cutler.[3] A team of six workers was assembled, and for 365 days without a break they counted every day the ciliates, the amœbæ, the flagellates, and the bacteria in a plot of arable ground, distinguishing no less than seventeen different kinds of protozoa. The conclusions arrived at were carefully tested by the Statistical Department.
Of the protozoa the flagellates were found to be the most numerous, the amœbæ came next, and the ciliates were by far the fewest. The numbers of each organism varied from day to day in a way that showed conclusively the essentially trophic nature of the protozoan population. The numbers of amœbæ—especially Dimastigamœba and of a species called α—were sharply related to the numbers of bacteria: when the amœbae were numerous the bacteria were few, and vice versa. Detailed examination showed that the amœbæ were probably the cause of the fluctuations in the bacterial numbers, but Mr. Cutler has not yet been able to find why the amœbæ fluctuated; it does not appear that temperature, moisture[11] content, air supply or food supply were determining causes. The flagellates and ciliates also showed large fluctuations, amounting in one case—Oicomonas—to a definite periodicity, apparently, however, not related to bacterial numbers, or, so far as can be seen, to external conditions of moisture, temperature and food supply, and showing no agreement with the fluctuations of the amœbæ. However, one cannot be certain that lack of agreement between curves expressing protozoan numbers and physical factors implies absence of causal relationships: the observations (though the best that can yet be made) are admittedly not complete. If we saw only the end of the bough of a tree, and could see no connection with a trunk, we might have much difficulty in finding relationships between its motion and the wind; whatever the direction of the wind it would move backwards and forwards in much the same way, and even when the wind was blowing along the plane of its motion it would just as often move against the wind as with it.
Meanwhile evidence was obtained that the twenty-four hour interval adopted by the protozoological staff was too long for bacteria, and accordingly the Bacteriological Department, under Mr. Thornton, refined the method still further. Bacterial counts were made every two hours, day and night, for several periods of sixty or eighty hours without a break. The shape of the curve suggests that two hours is probably close enough, and for the present counts at shorter intervals are not contemplated. But there is at least one maximum and one minimum in the day, although the bacterial day does not apparently correspond with ours, nor can any relationship be traced with the diurnal temperature curve.
The nitrate content of the soil was simultaneously determined by Mr. Page and found to vary from hour to hour, but the variations did not sharply correspond with the bacterial numbers; this, however, would not necessarily be expected. The production of nitrate involves various stages, and any lag would throw the nitrate and bacterial curves out of agreement. There is a suggestion of a lag,[12] but more counts are necessary before it can be regarded as established.
Examination of these and other nitrate curves obtained at Rothamsted has brought out another remarkable phenomenon. No crop is growing on these plots, and no rain fell during the eighty hours, yet nitrate is disappearing for a considerable part of the time. Where is it going to? At present the simplest explanation seems to be that it is taken up by micro-organisms. A similar conclusion had to be drawn from a study of the nitrogen exhaustion of the soil. The whole of the nitrate theoretically obtainable from the organic matter of the soil is not obtained in the course of hours or even days; in one of our experiments at Rothamsted nitrification is still going on, and is far from complete, even after a lapse of fifty-three years. The explanation at present offered is that part of the nitrate is constantly being absorbed by micro-organisms and regenerated later on.
Now what organisms could be supposed to absorb nitrates from the soil? Certain bacteria and fungi are known to utilise nitrates, and one naturally thinks of algæ as possible agents also. Dr. Muriel Bristol was therefore invited to study the algæ of the soil. Her account is given in Chapter VI. She has found them not only on the surface, but scattered throughout the body of the soil, even in the darkness of 4 inches, 5 inches, or 6 inches depth, where no light can ever penetrate, and where photosynthesis as we understand it could not possibly take place. Some modification in their mode of life is clearly necessary, and it may well happen that they are living saprophytically. Dr. Bristol has not yet, however, been able to count the algæ in the soil with any certainty, although she has made some estimates of the numbers.
The quantitative work on the soil population indicates other possibilities which are being investigated. There is not only a daily fluctuation in the numbers, but so far as measurements have gone, a seasonal one also. There seems to be some considerable uplift in numbers of bacteria, protozoa, and[13] possibly algæ and fungi in the spring-time, followed by a fall in summer, a rise in autumn, and a fall again in winter. At present we are unable to account for the phenomenon, nor can we be sure that it is general until many more data are accumulated.
In the cases of the protozoa and the algæ, there was a definite reason for seeking them in the soil.
Another section of the population, the fungi, was simply found, and at present we have only limited views as to their function. The older workers considered that they predominated in acid soils, while bacteria predominated in neutral soils. Present-day workers have shown that fungi, including actinomycetes, are normal inhabitants of all soils. The attempts at quantitative estimations are seriously complicated by the fact that during the manipulations a single piece of mycelium may break into fragments, each of which would count as one, while a single cluster of spores might be counted as thousands. Little progress has therefore been made on the quantitative lines which have been so fruitful with protozoa. Dr. Brierley gives, in Chapters VII. and VIII., a critical account of the work done on fungi.
In addition to the organisms already considered there are others of larger size. The nematodes are almost visible to the unaided eye, most of them are free living and probably help in the disintegration of plant residues, though a few are parasitic on living plants and do much injury to clover, oats, and less frequently to onions, bulbs, and potatoes. Further, there are insects, myriapods and others, the effects of which in the soil are not fully known. Special importance attaches to the earthworms, not only because they are the largest in size and in aggregate weight of the soil population, but because of the great part they play in aerating the soil, gradually turning it over and bringing about an intimate admixture with dead plant residues, as first demonstrated by Darwin. Earthworms are the great distributors of energy material to the microscopic population. Systematic quantitative work on these larger forms is only of recent date, and Dr. Imms, in Chapter IX., discusses our present knowledge.
[14]
TABLE I.
Soil Population, Rothamsted, 1922.
(The figures for algæ and fungi are first approximations only, and have considerably less value than those for bacteria and protozoa.)
| Numbers per Gram of Soil. |
Approximate Weight per Acre of— |
|||||||
|---|---|---|---|---|---|---|---|---|
| Living Organisms. |
Dry Matter in Organisms. |
Nitrogen in Organisms. |
||||||
| Bacteria— | lb. | lb. | lb. | |||||
| High level | 45,000,000 | 50 | } | 2 | 0·2 | |||
| Low level | 22,500,000 | 25 | ||||||
| Protozoa— | ||||||||
| Ciliates— | ||||||||
| High level | 1,000 | — | — | — | ||||
| Low level | 100 | — | — | — | ||||
| Amœbæ— | ||||||||
| High level | 280,000 | 320 | } | 12 | 1·2 | |||
| Low level | 150,000 | 170 | ||||||
| Flagellates— | ||||||||
| High level | 770,000 | 190 | } | 7 | 0·7 | |||
| Low level | 350,000 | 85 | ||||||
| Algæ (not blue-green) | [100,000 | ] | 125 | 6 | 0·6 | |||
| Blue-green | Not known. | — | Say 6 | Say 0·6 | ||||
| Fungi— | ||||||||
| High level | [1,500,000 | ] | 1700 | } | 60 | 6·0 | ||
| Low level | [700,000 | ] | 800 | |||||
| 93 | 9·3 | |||||||
| = 4 parts nitrogen per 1,000,000 of soil. |
||||||||
| Larger Organisms. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Numbers per Acre.[D] |
Approximate Weight per Acre of— |
|||||||
| Living Organisms. |
Dry Matter in Organisms. |
Nitrogen in Organisms. |
||||||
| Ma- nured. |
Un- ma- nured. |
Ma- nured. |
Un- ma- nured. |
Ma- nured. |
Un- ma- nured. |
Ma- nured. |
Un- ma- nured. |
|
| Oligochaeta (Limicolae)— | lb. | lb. | lb. | lb. | lb. | lb. | ||
| Nematoda, etc. | 3,609,000 | 794,000 | 9 | 2 | 3 | 1 | — | — |
| Myriapoda | 1,781,000 | 879,000 | 203 | 99 | 85 | 42 | 4 | 2 |
| Insects | 7,727,000 | 2,475,000 | 34 | 16 | 14 | 6 | 1 | 1 |
| Earthworms | 1,010,000 | 458,000 | 472 | 217 | 108 | 50 | 10 | 5 |
| Total | 210 | 99 | 15 | 9 | ||||
| Total organic matter (dry weight) in this soil = 126,000 lb. per acre. | ||||||||
| Total nitrogen = 5700 lb. per acre. (1 lb. nitrogen per acre = 0·4 parts per 1,000,000 of soil.) | ||||||||
| [D] To a depth of 9 inches. The weight of soil is approximately 1,000,000 kilos. | ||||||||
[15]
Are there any other members of the soil population that are of importance? As already shown, the method of investigating the soil population in use at Rothamsted is to find by chemical methods the changes going on in the soil; to find by biological methods what organisms are capable of bringing about these changes; and then to complete the chain of evidence by tracing the relationships between the numbers or activities of these organisms and the amount of change produced. The list as we know it to-day is given in Table I.
The method, however, does not indicate whether the account is fairly complete, or whether there are other organisms to be found. We might, of course, trust to empirical hunting for organisms, or to chance discoveries such as led Goodey to find the mysterious Proteomyxan Rhizopods, which cannot yet be cultured with certainty, so that they are rarely found by soil workers. It is possible that there are many such organisms, and it is even conceivable that these unknown forms far outnumber the known. The defect of the present method is that it always leaves us in doubt as to the completeness of the list, and so we may have to devise another.
Reverting to Table I., it obviously serves no purpose to add the numbers of all the organisms together. We can add up the weights of living organisms, of their dry matter or nitrogen, so as to form some idea of the proportion of living to non-living organic matter, and this helps us to visualise the different groups and place them according to their respective masses. But a much better basis for comparing the activities of the different groups would be afforded by the respective amounts of energy they transform, if these could be determined. It is proposed to attempt such measurements at Rothamsted. The results when added would give the sum of the energy changes effected[16] by the soil population as we know it: the figure could be compared with the total energy change in the soil itself as determined in a calorimeter. If the two figures are of the same order of magnitude, we shall know that our list is fairly well complete; if they are widely different, search must be made for the missing energy transformers. There are, of course, serious experimental difficulties to be overcome, but we believe the energy relationships will afford the best basis for further work on the soil population.
Finally, it is necessary to refer to the physical conditions obtaining in the soil. These make it a much better habitat for organisms than one might expect. At first sight one thinks of the soil as a purely mineral mass. This view is entirely incorrect. Soil contains a considerable amount of plant residues, rich in energy, and of air and water. The usual method of stating the composition of the soil is by weight, but this is misleading to the biologist because the mineral matter has a density some two and a half times that of water and three times that of the organic matter. For biological purposes composition by volume is much more useful, and when stated in this way the figures are very different from those ordinarily given. Table II. gives the results for two Broadbalk arable plots, one unmanured and the other dunged; it includes also a pasture soil.
The first requirement of the soil population is a supply of energy, without which it cannot live at all. All our evidence shows that the magnitude of the population is limited by the quantity of energy available. The percentage by weight of the organic matter is about two to four or five, and the percentage by volume runs about four to twelve. Not all of this, however, is of equal value as source of energy. About one-half is fairly easily soluble in alkalis, and may or may not be of special value, but about one-quarter is probably too stable to be of use to soil organisms.
[17]
A second requirement is water with which in this country the soil is usually tolerably well provided. Even in prolonged dry weather the soil is moist at a depth of 3 inches below the surface. It is not uncommon to find 10 per cent. or 20 per cent. by volume of water present, spread in a thin film over all the particles, and completely saturating the soil atmosphere.
TABLE II.
Volume of Air, Water and Organic Matter in 100 Volumes of
Rothamsted Soil.
| Solid Matter. | Pore Space. |
In Pore Space. Values Commonly Obtained. |
|||
|---|---|---|---|---|---|
| Mineral. | Organic. | Water. | Air. | ||
| (1) | 62 | 4 | 34 | 23 | 11 |
| (2) | 51 | 11 | 38 | 30 | 8 |
| (3) | 41 | 12 | 47 | 40 | 7 |
(1) Arable, no manure applied to soil. (2) Arable, dung applied to soil. (3) Pasture.
The air supply is usually adequate owing to the rapidity with which diffusion takes place. Except when the soil is water-logged, the atmosphere differs but little from that of the one we breathe. There is more CO2, but only a little less oxygen.[8] The mean temperature is higher than one would expect, being distinctly above that of the air, while the fluctuations in temperature are less.[5]
The reaction in normal soils is neutral to faintly alkaline; pH values of nearly 8 are not uncommon. Results from certain English soils are shown on p. 18.
The soil reaction is not easily altered. A considerable amount of acid must accumulate before any marked increase in intensity of pH value occurs; in other words, the soil is well buffered. The same can be said of temperature, of water, and of energy supply. Like the reaction, they alter[18] but slowly, so that organisms have considerable time in which to adapt themselves to the change.
Hydrogen Ion Concentration and Soil Fertility.
| pH | |||||
|---|---|---|---|---|---|
| Alkaline | 10 | Sterile: Alkali soil. | |||
| 9 | |||||
| 8 | Fertile: Arable. | ||||
| Neutral | 7 | ||||
| 6 | |||||
| 5 | Potato Scab fails. Nitrification hindered. Barley fails. |
||||
| 4 | |||||
| Acid | 3 | Sterile: Peat. | |||
[1] Berthelot, Marcellin, “Fixation directe de l’azote atmosphérique libre par certains terrains argileux,” Compt. Rend., 1885, ci., 775-84.
[2] Boussingault, J. B., and Léwy, “Sur la composition de l’air confiné dans la terre végétale,” Ann. Chim. Phys., 1853, xxxvii., 5-50.
[3] Cutler, D. W., Crump, L. M., and Sandon, H., “A Quantitative Investigation of the Bacterial and Protozoan Population of the Soil, with an Account of the Protozoan Fauna,” Phil. Trans. Roy. Soc., Series B, 1922, ccxi., 317-50.
[4] Hellriegel, H., and Wilfarth, H., “Untersuchungen über die Stickstoffnahrung der Gramineen und Leguminosen,” Zeitsch. des Vereins f. d. Rübenzucker-Industrie, 1888.
[5] Keen, B. A., and Russell, E. J., “The Factors determining Soil Temperature,” Journ. Agric. Sci., 1921, xi., 211-37.
[6] Lawes, J. B., and Gilbert, J. H., “On Agricultural Chemistry, Especially in Relation to the Mineral Theory of Baron Liebig,” Journ. Roy. Agric. Soc., 1851, xii., 1-40.
[7] Liebig, Justus, “Chemistry in its Application to Agriculture and Physiology,” 1st and 2nd editions (1840 and 1841), 3rd and 4th editions (1843 and 1847); “Natural Laws of Husbandry,” 1863.
[8] Russell, E. J., and Appleyard, A., “The Composition of the Soil Atmosphere,” Journ. Agric. Sci., 1915, vii., 1-48; 1917, viii., 385-417.
[19]
[9] Russell, E. J., and Hutchinson, H. B., “The Effect of Partial Sterilisation of Soil on the Production of Plant Food,” Journ. Agric. Sci., 1909, iii., 111-14; Part II., Journ. Agric. Sci., 1913, v., 152-221.
[10] Schloesing, Th., and Müntz, A., “Sur la Nitrification par les ferments organisés,” Compt. Rend., 1877, lxxxiv., 301-3; 1877, lxxxv., 1018-20; and 1878, lxxxvi., 892-5. “Leçons de chimie agricole,” 1883.
[11] Warington, R., “On Nitrification,” Part I., Journ. Chem. Soc., 1878, xxxiii., 44-51; Part II, Journ. Chem. Soc., 1879, xxxv., 429-56; Part III., Journ. Chem. Soc., 1884, xlv., 637-72; Part IV., Journ. Chem. Soc., 1891, lix., 484-529.
[12] Way, J. T., “On the Composition of the Waters of Land Drainage and of Rain,” Journ. Roy. Agric. Soc., 1856, xvii., 123-62.
[13] Winogradsky, S., “Recherches sur les organismes de la nitrification,” Ann. de l’Inst. Pasteur, 1890, iv., 1e Mémoire, 213-31; 2e Mémoire, 257-75; 3e Mémoire, 760-71.
“Recherches sur l’assimilation de l’azote libre de l’atmosphère par les microbes.” Arch. des Sci. Biolog. St. Petersburg, 1895, iii, 297-352.
For further details and fuller bibliography, see E. J. Russell, “Soil Conditions and Plant Growth,” Longmans, Green & Co.
[20]
To understand the development of our knowledge of soil bacteria, it must be remembered that bacteriology is under the disadvantage that it started as an applied science. Although bacteria were first seen by Leeuwenhoeck about the middle of the seventeenth century, and some of their forms described by microscopists of the eighteenth and early nineteenth centuries, it was only with the work of Pasteur on fermentation, and of Duvaine, Pasteur, and their contemporaries on disease bacteria, that bacteriology may be said to have started. From the outset, therefore, attention has been directed mainly to the bacteria in their specialised relationship to disease or to fermentation and similar processes. As a result, little research was done on the pure biology of the bacteria, so that even now many of the most fundamental and elementary problems concerning them are quite unsolved.
In their work on fermentations and disease bacteria, the earlier workers were assisted by the fact that under both sets of conditions the causative bacteria exist, as a rule, either in practically pure culture, or else in preponderating numbers. The study and elucidation of such a mixed micro-population as exists in the soil, became possible only when methods had been devised for isolating the different kinds of bacteria, and thus studying them apart from each other. It was the development of the gelatine plate method of isolating pure cultures by Koch[36] in 1881 that made the study of the soil bacteria practicable. The plating method[21] opened up two lines of research. In the first place, it provided a simple means of isolating organisms from the mixed population of the soil, and thus enabled a qualitative study to be made of each organism in pure culture, and, in the second place, from it was developed a counting technique for estimating differences in bacterial numbers between samples of soil, from which has sprung much of our knowledge of the quantitative side.
The earliest studies of the soil bacteria consisted of such estimations of numbers, and showed that the soil contained a very numerous population of bacteria. About 20,000,000 bacteria per gram of soil is now considered a fair average number. The number and variety of bacteria existing in the soil is so enormous that the method of separating out all the different forms, and of discovering their characters and functions, has proved impracticable. In practice, therefore, the problem has been approached from the biochemical standpoint. That is to say, the special chemical changes that the bacteria produce in the soil have first been investigated, and this has been followed by the isolation and study of the various groups of bacteria that bring about the changes under investigation.
The method commonly employed in isolating the organisms that produce a given chemical change in the soil is called the “elective” method. The soil is inoculated into a culture medium that will especially favour the group of bacteria to be isolated, to the exclusion of others. For example, if it is desired to isolate the organisms that attack cellulose, a medium is made up containing no other organic carbon compounds except cellulose. Such a selective medium encourages the growth of the group of organisms to be investigated, so that after several transfers to fresh medium a culture is obtained containing only two or three different types of organisms. These are separated by plating and pure cultures obtained.
Another difficulty which has not yet been completely overcome is that of adequately describing an organism when[22] it is isolated. The morphology of bacteria is not the constant thing that is seen in the more stable higher organisms. In many cases the appearance of a single strain is entirely different on different media, and may be quite altered by such conditions as changes in acidity of the medium or temperature of incubation. Even on a single medium remarkable changes in morphology occur, at any rate, in some bacteria. This is well seen in a cresol-decomposing organism under investigation at Rothamsted. In cultures a few days old this organism develops as bent and branching rods; these rods then break up into chains of cocci and short rods, which separate, and in old cultures all the organisms may be in the coccoid form (Fig. 1). It is claimed by Löhnis[47b] that the possession of a complex life-cycle of changing forms is a universal character in the bacteria. The instability of shape in many bacteria makes it necessary to standardise very carefully the cultural conditions under which they are kept when their appearance is described.

Culture 15 hours old. Culture 3 days old.
Fig. 1.—Change in appearance, in culture, of a cresol decomposing bacterium.
The inadequacy of mere morphology as a basis for describing bacteria led to the search for diagnostic characters, based on the biochemical changes that they produced in their culture media, and the appearance of their growth in the mass on various media. These characters unfortunately have also proved to be very much influenced by the exact composition of the medium and other conditions of culture.[23] Recently an attempt has been made by the American Society of Bacteriologists to standardise the diagnostic characters used in describing bacteria, and also the media and cultural conditions under which they are grown for the purpose of description. The need for such precautions, however, was not sufficiently realised by the early workers, many of whose descriptions cannot now be referred to any definite organism.
The large number of organisms found in the soil, and the difficulty and labour of adequately describing them, is such that even now we have no comprehensive description of the common soil bacteria that appear on gelatine platings. A careful study based on modern methods of characterisation has been made of certain selected groups of bacteria, and it is hoped that the laborious systematic work of describing the common forms will gradually be completed.
Several attempts have been made to classify the bacteria that appear commonly on gelatine platings. This work was commenced by Hiltner and Stormer in Germany, and continued by Chester, Harding, and Conn in America. Conn[10], [14] found that the common organisms fell into the following main groups:—
(1) Large spore-forming bacteria, related to Bacillus subtilis, which form about 5-10 per cent. of the numbers. He adduced evidence[12], [13] that these organisms exist in the soil mainly as spores, so that they may not form an important part of the active soil population.
(2) Short non-sporing organisms, related to Pseudomonas fluorescens, that are rapid gelatine liquefiers. These form another 10 per cent. of the numbers.
(3) Short rod forms that liquefy gelatine slowly or not at all, and develop colonies very slowly. These form 40-75 per cent. of the numbers, and may therefore be of considerable importance in the soil.
(4) A few micrococci also occur.
These groups comprise the larger portion of the bacterial flora of the soil, but, in addition to these organisms, that develop on the media commonly used for plating, there are[24] special and important groups that appear only on special media, either owing to their being unable to grow on ordinary media or because they get swamped by other forms. Examples of such groups are the ammonia and nitrite oxidising bacteria, the nitrogen fixing groups, the cellulose decomposing organisms, and the sulphur bacteria.
In order that we may apply the results of the study of a definite organism to other localities, a knowledge of the geographical distribution of the soil bacteria is clearly needed. We have, unfortunately, very little knowledge of the distribution of soil organisms. The common spore-forming groups appear to be universally distributed. Thus Barthel, in a study of the bacterial flora of soils from Greenland and the island of Disko, obtained soil organisms belonging to the groups of Bacillus subtilis, B. amylobacter, B. fluorescens, B. caudatus, and B. Zopfii, which are common groups in European soil, indicating that the general constitution of the bacterial flora of the soil in arctic regions is not widely different from that of Western Europe. Bredemann, who made an extensive study of the Bacillus amylobacter group, obtained soil samples from widely scattered localities, and found these organisms in soil from Germany, Holstein, Norway, Italy, Morocco, Teneriffe, Russia, Japan, China, the East Indies, Samoa, Illinois, Arizona, German East Africa, and the Cameroons. Some soil organisms, on the other hand, are apparently absent from certain districts. This may be due to the conditions, such as climatic environment, being unfavourable to them. A study has recently been made at Rothamsted of the distribution over Great Britain of a group of bacteria that are capable of decomposing phenol and cresol. One of these organisms, apparently related to the acid-fast B. phlœi, has an interesting distribution. It has been found in 50 per cent. of the soils samples examined from the drier region, where the annual rainfall is less than 30 inches, but in only 20 per cent. of the samples in the wetter parts of Britain. Another example of limited distribution is found in the case of Bacillus radicicola,[25] the organism that produces tubercles on the roots of leguminous plants. The distribution of the varieties of this organism follows that of the host plants with which they are associated, so that when a new leguminous crop is introduced into a country, nodules may not appear on the roots unless the soil be specially inoculated with the right variety of organism. In cases where a group of soil organisms is widely distributed over the globe, it may yet be absent from many soils owing to the soil conditions not suiting it. Thus, phenol decomposing bacteria, though abundant in the neighbourhood of Rothamsted, are yet absent from field plots that have been unmanured for a considerable period. The occurrence of the nitrifying organisms and the nitrogen fixing Azotobacter is also very dependent on the soil conditions.
Owing to the method by which our knowledge of soil bacteria has been acquired, by studying first the chemical changes in the soil and then the bacteria that produce them, it is natural for us to divide them into physiological groups according to the chemical changes that they bring about. This grouping is the more reasonable since so little is known as to the true relationships of the different groups of bacteria that a classification based on morphology is well-nigh impossible. In considering the activities of bacteria in the soil, it is convenient to group the changes which they bring about into the two divisions into which they naturally fall in the economy of the organisms.
In the first place, there are the changes that result in a release of energy, which the bacteria utilise for their vital processes.
In the second place, there are the processes by which the bacteria build up the material of their bodies. These building up processes involve an intake of energy for their accomplishment.
It will be convenient to deal first with the release of energy for their own use by bacteria, and its consequences.
[26]
Unlike the green plants, most bacteria are unable to obtain the energy that is required for their metabolism from sunlight. They must, therefore, make use of such chemical changes as will involve the release of energy.
As an example of the acquirement of energy in this way may be taken the oxidation of methane by B. methanicus. This organism, described by Söhngen, obtains its energy supply by the conversion of methane into CO2 and H2O.
CH4 + 2O2 = CO2 + 2H2O 220 Cal.
A further example is the acetic organism that obtains its energy through the oxidation of alcohol to acetic acid.
C2H6O + O2 = C2H4O2 + H2O 115 Cal.
The decomposition processes brought about by micro-organisms in obtaining energy are usually oxidations, but this is not necessarily so, as can be seen in case of the fermentation of sugar into alcohol.[E]
C6H12O6 = 2C2H6O + 2CO2 50 Cal.
[E] These examples are from Orla-Jensen (Centralblatt f. Bakt., II., Bd. 22, p. 305).
By far the greater part of the decomposition of organic matter is brought about by bacteria in the process of acquiring energy. In the soil, nearly the whole of the material utilised by bacteria as a source of energy is derived ultimately from green plants. The energy materials left in the soil by the plant fall into two groups, the non-nitrogenous compounds, which are mainly carbohydrates and their derivatives, and the nitrogenous compounds, principally derived from proteins.
The simpler carbohydrates and starches are attacked and decomposed by a large variety of bacteria. The addition[27] of such substances to soil causes a rapid increase in bacterial numbers. In nature the sugars are in all probability among the first plant constituents to be destroyed during the decay processes.
A large proportion of plant tissues consist of cellulose and its derivatives. These compounds are consequently of great importance in the soil. Unfortunately our knowledge of the processes by which cellulose is broken down in the soil is very inadequate. The early experimental study of cellulose decomposition, such as that of Tappeiner[60] and Hoppe-Seyler,[33] was mostly carried out under conditions of inadequate aeration, and the products of decomposition were found to include methane and CO2, and sometimes fatty acids and hydrogen. The bacteriology of this anaerobic decomposition was studied by Omelianski,[54] who described two spore-bearing organisms, one of which attacked cellulose with the production of hydrogen, and the other with the production of methane. Both species also produce fatty acids and CO2. It is probable that these organisms operate in the soil under conditions of inadequate aeration. In swamp soils, in which rice is grown, it has been shown that methane, hydrogen, and CO2 are evolved in the lower layers. In these soils, however, the methane and hydrogen are oxidised when they reach the surface layers. This oxidation is also effected by micro-organisms. Bacteria capable of deriving energy by the oxidation of hydrogen gas have been isolated and studied by Kaserer,[37] and by Nabokich and Lebedeff,[52] while Söhngen[57] has isolated an organism which he named Bacillus methanicus, that was capable of oxidising methane.
Under normal conditions in cultivated soils, however, the decomposition of cellulose takes place in the presence of an adequate air supply, and so follows a different course from that studied by Omelianski. Our knowledge of this aerobic decomposition is very scanty. A number of bacteria, capable of decomposing cellulose aerobically, are known. A remarkable organism was investigated by Hutchinson and[28] Clayton,[30] who named it Spirochæta cytophaga. This organism, which they isolated from Rothamsted soil, though placed among the Spirochætoidea, is of doubtful affinities. During the active condition it exists for the most part as thin flexible rods tapered at the extremities. This form passes into a spherical cyst-like stage, at first thought to be a distinct organism (Fig. 2). Spirochæta cytophaga is very aerobic, working actively, only at the surface of the culture medium. It is very selective in its action. It appears unable to derive energy from any carbohydrate other than cellulose. Indeed, many of the simple carbohydrates, especially the reducing sugars, are toxic to the organism in pure culture. An extensive study of aerobic cellulose decomposition by bacteria was made by McBeth and Scales,[50] who isolated fifteen bacteria having this power. Five of these were spore-forming organisms. Unlike Spirochæta cytophaga, they are all able to develop on ordinary media such as beef agar or gelatine, and are thus not nearly so selective in their food requirements.

Fig. 2.—Spirochæta cytophaga. Changes occurring in culture. (After Hutchinson and Clayton.)
We are at present ignorant as to which organisms are most effective in decomposing cellulose in the soil under field conditions, or what are the conditions best suited to their activity. It is possible that fungi also help in the decomposition of cellulose to a great extent. This subject of the decomposition of cellulose offers one of the most promising fields of research in soil bacteriology. The difficulty of the subject is further increased by our present ignorance of the chemical aspect of cellulose decomposition. It has been supposed that the early decomposition products are simpler sugars, but these are not found under conditions[29] in which cellulose is being decomposed by pure cultures of the bacteria mentioned above. Hutchinson and Clayton found that their organism produced volatile acids, mucilage, and a carotin-like pigment. The organisms isolated by McBeth and Scales also produce acids, and in some cases yellow pigments. It is known, however, that the decomposition products of cellulose can be utilised as energy supply for other organisms, such as nitrogen fixing bacteria.
When plant remains decompose in the soil there are ultimately produced brown colloidal bodies collectively known as humus. The processes by which this humus is produced are not yet properly understood. Humus is of great importance in the soil, in rendering the soil suitable for the growth of crops. It affects the physical properties of the soil to a great extent. In the first place, it improves the texture of the soil, making heavy clay soils more friable, and loose sandy soils more coherent. Secondly, it has great water-retaining powers, so that soils rich in organic matter suffer comparatively little during periods of drought. And lastly, it exerts a strong buffering effect against soil acids. Now, it is one of the problems of present-day farming that soil is becoming depleted of its humus. This is due to the increasing scarcity of farmyard manure in many districts, and the consequent use of mineral fertilisers to supply nitrogen, potash, and phosphate to the crop. A need has therefore arisen for a substitute for farmyard manure, by means of which the humus content of soils may be kept up in districts where natural manure is scarce.
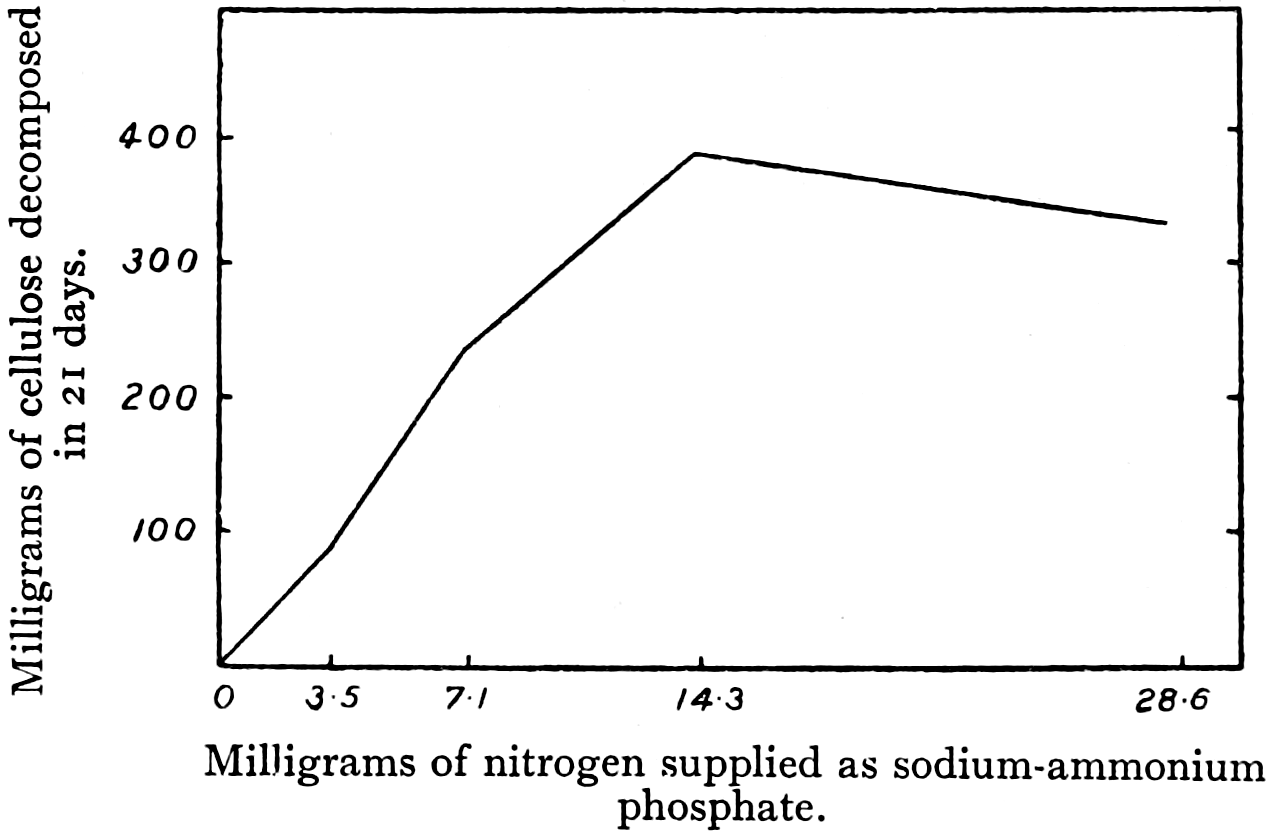
Fig. 3.—Cellulose decomposed by S. cytophaga in media with increasing amounts of nitrogen. (After Hutchinson and Clayton.)
X-axis: Milligrams of nitrogen supplied as sodium-ammonium phosphate.
Y-axis: Milligrams of cellulose decomposed in 21 days.
It is well known that if fresh unrotted manure or straw be added to the soil, it often produces harmful effects on the succeeding crop. The problem, therefore, was to develop a method by which fresh straw, before application to the soil, could be made to rot down to a mixture of humus compounds such as occur in well-rotted farmyard manure. The solution of this problem came as a result of an investigation by Hutchinson and Richards,[30b] at Rothamsted, into food requirements of the cellulose decomposing bacteria. They[30] realised that since more than 10 per cent. of the dry weight of bacteria consists of nitrogen, it would be necessary to supply the cellulose decomposing bacteria with a supply of nitrogen, in order that they should attain their greatest activity. Experiments with cultures of Spirochæta cytophaga showed that the amount of cellulose decomposed depended upon an adequate supply of nitrogen for the organism (Fig. 3). Similarly, materials such as straw will scarcely decompose at all if wetted with pure water. An adequate supply of nitrogen compounds is needed to enable decomposition to take place. Hutchinson and Richards tested the effect of ammonium sulphate, and discovered experimentally the proportion of ammonia to straw that produced the most rapid decomposition. They found that if a straw heap was treated with the correct proportion of ammonia, it decomposed into a brown substance having the appearance of well-rotted manure. This has resulted in the development of a commercial process for making synthetic farmyard manure from straw. The method of manufacture is as follows: A straw stack is made and thoroughly wetted with water. The correct amount of ammonium sulphate is then sprinkled on the top and wetted, so that the solution percolates through[31] the straw. The cellulose bacteria attack the straw, breaking it down and assimilating the ammonia. This ammonia is not wasted, as it is converted into bacterial protoplasm that eventually decays in the soil. Field trials of this synthetic manure show that it produces an effect closely similar to that of natural farmyard manure.
While cellulose and related carbohydrates are by far the most important non-nitrogenous compounds left in the soil by plants, there are other compounds whose destruction by bacteria is of special interest. Such, for example, is the case of phenol. This compound is produced by bacterial action as a decomposition product of certain amino-acids. It occurs in appreciable amounts in cow urine. It is probable that it forms a common decomposition product in soil and also in farmyard manure. If this phenol were to persist in the soil, it would eventually reach a concentration harmful to plant growth. It does not, however, accumulate in the soil; indeed, if pure phenol or cresol be added to ordinary arable soil, a rapid disappearance occurs. This disappearance is of some practical importance, since it limits the commercial use of these compounds as soil sterilising agents. The cause of the disappearance has been to some extent elucidated at Rothamsted,[58] where it was found to be in part a purely chemical reaction with certain soil constituents, and partly due to the activity of bacteria capable of decomposing it. A large number of soil bacteria have now been isolated that can decompose phenol, meta-, para-, and ortho-cresol, and are able to use these substances as the sole sources of energy for their life processes. These organisms have a wide distribution, having been found in soil samples taken from all over Great Britain, from Norway, the Tyrol, Gough Island, Tristan da Cunha and South Georgia. Soil bacteria have also been isolated that are able to decompose and derive their energy from naphthalene and from toluene. The ability of the bacteria to break up the naphthalene is very remarkable, and all the more so since they can hardly have come across this compound[32] in the state of nature. The naphthalene organisms have a distribution as world-wide as the phenol group.
The second main group of products left in the soil by higher plants are the nitrogen-containing compounds, such as the proteins and amino-acids. Plant remains are not the only source of organic nitrogen compounds available to soil bacteria. There are, in addition, the dead bodies of other soil organisms, such as protozoa and algæ. The relative importance of these sources of nitrogen is not known, but almost certainly varies greatly with the state of activity of the various groups of the soil population. Bacteria are able to utilise organic nitrogen compounds as energy sources, as can be exemplified in the oxidation of a simple amino-acid:—
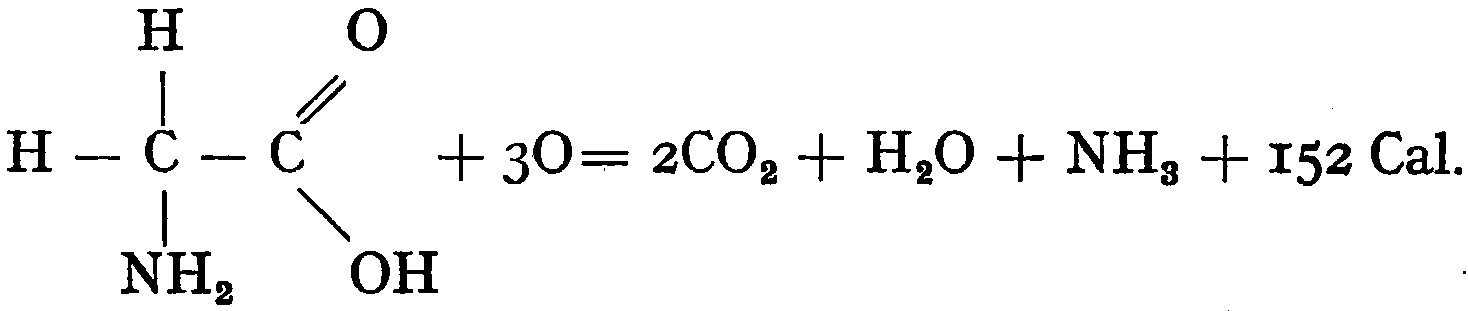
It will be seen that, in the acquirement of energy from such a compound, ammonia is released as a by-product. It is not certainly known what is the exact course of the reactions brought about by bacteria in soil during the breaking-down of organic nitrogen compounds, but they result in the splitting off of most of the nitrogen as ammonia. Herein lies the great importance of the process, for the production of ammonia is an essential stage in the formation of nitrate in the soil, and on the supply of nitrate the growth of most crops largely depends.
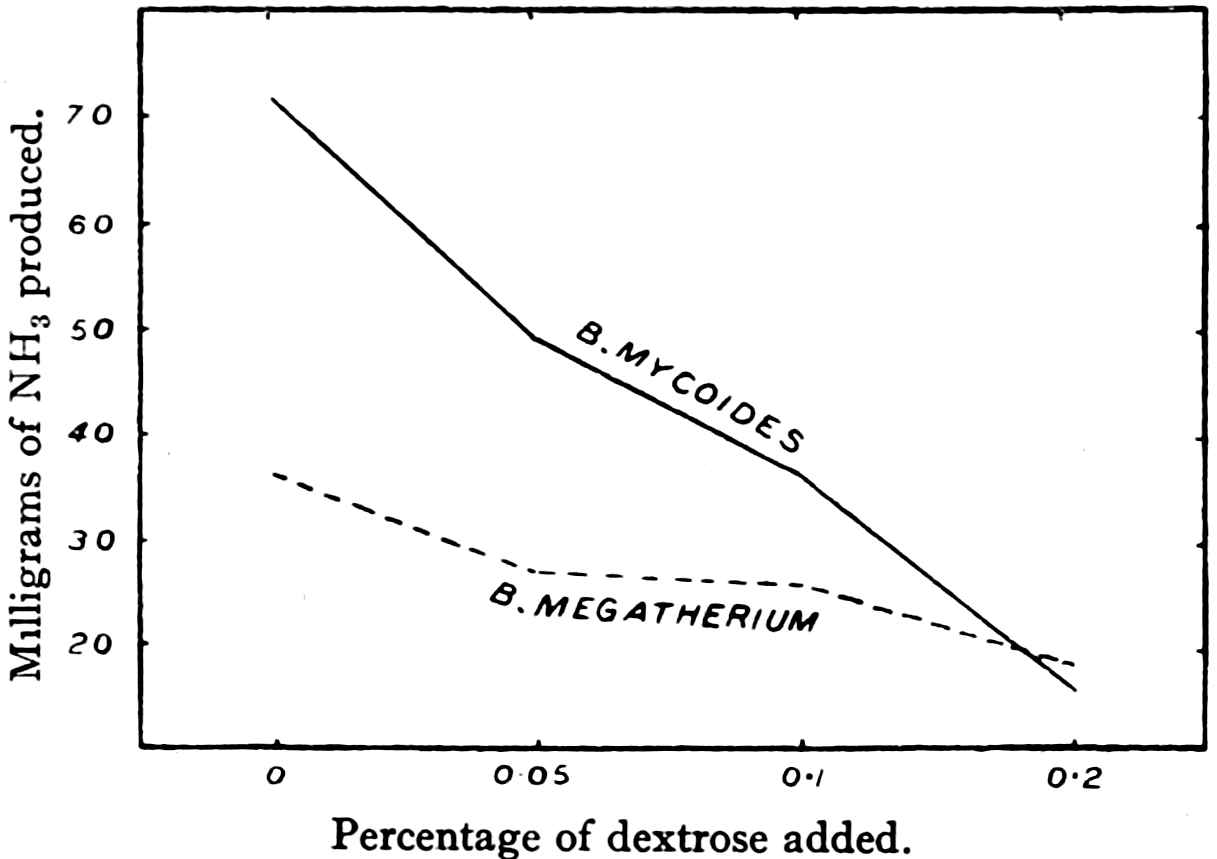
Fig. 4.—Quantities of ammonia produced by pure cultures from 5 grams of casein in the presence of varying quantities of dextrose. (After Doryland.)
X-axis: Percentage of dextrose added.
Y-axis: Milligrams of NH3 produced.
It is very important to note that the production of this ammonia is only a by-product in the economy of the bacteria, the benefit that they derive from the reactions being due to the release of energy involved in the decomposition. The common ammonia-producing bacteria in the soil have been found equally capable of deriving their energy by the oxidation[33] of sugars and similar non-nitrogenous compounds. Fig. 4 shows an experiment by Doryland,[17] in which cultures of common soil bacteria were grown in peptone solution, to which increasing quantities of sugar were added. One can see that, as the amount of sugar is increased, the production of ammonia is lowered, since the bacteria are obtaining energy from the sugar instead of from the nitrogen compound, peptone. Consequently, if soil contains a quantity of easily decomposible carbohydrate material, bacteria will derive their energy from this source, and the production of ammonia and nitrate will be lowered. Thus the addition of sugar or unrotted straw to the soil often lowers the nitrate production, and consequently reduces the crop yield. If the soil is sufficiently rich in carbohydrate material, the bacteria may multiply until the supply of organic nitrogen is used up, and then will actually assimilate some of the ammonia and nitrate already existing. There is thus a balance of conditions in the soil due to varying proportions of nitrogenous and non-nitrogenous energy material. When nitrogen compounds are the predominant energy source, the bacteria utilise them, and[34] ammonia is released. When a non-nitrogenous energy source predominates, this is utilised and little or no ammonia is released, and in extreme cases ammonia may be assimilated.
Although a large number of the common organisms in the soil produce ammonia in culture media containing peptone, the relative importance of these in the soil has yet to be decided. It was supposed that the spore-forming organisms related to Bacillus mycoides were of chief importance. This supposition dates from the work of Marchal,[49] who studied the production of ammonia by an organism of this group in culture solution, and found it to be a very active ammonifier. As already mentioned, however, there is some doubt as to whether the large spore-forming organisms are very active under soil conditions.[12], [13] The existence of rapid fluctuations in nitrate content, found to exist in soil, may in the future indicate which are the most active of the common bacteria in the soil itself by enabling us to observe which types increase during periods of rapid ammonia and nitrate formation.
The ammonia produced in the soil under normal field conditions is rapidly oxidised successively to nitrite and to nitrate, a process known as nitrification. The process of nitrification is more rapid than that of ammonia production, with the consequence that no more than traces of ammonia are able to accumulate. The rate at which nitrate is formed in the soil is consequently set by the slower process of ammonia production.
The work of Schloesing and of Warington showed that the oxidation of ammonia was the work of living organisms. It is, however, to Winogradsky’s isolation and study of the causative organisms that we owe our present knowledge of the biology of the process. By a new and ingenious technique, he isolated from soil two remarkable groups of bacteria that bring about nitrification. The first group oxidises ammonium carbonate to nitrite, and was divided by[35] Winogradsky into the two genera, Nitrosomonas, a very short rod-like organism bearing a single flagellum, and Nitrosococcus, a non-motile form found in South America. The second group oxidises nitrites to nitrates. They are minute pear-shaped rods to which he gave the name Nitrobacter.
Winogradsky found that the first, or nitrite-producing group, would live in a culture solution containing:—
| 2·25 | grams | ammonium sulphate, |
| 2·0 | „ | sodium chloride, |
| 1·0 | „ | magnesium carbonate, |
| to the litre of well water. | ||
Nitrobacter would grow in a similar medium containing sodium nitrite instead of ammonium sulphate. There being no organic carbon in these media, the organisms had no source of carbon for their nutrition, except the CO2 of the air, or possibly that of bicarbonate in solution. It therefore followed that the organisms must obtain their carbon supply from one of these sources. Unlike green plants, the nitrous and nitric organisms are able to carry on this carbon assimilation in the dark, and must therefore obtain the energy needed for the process from some chemical reaction. The only sources of energy in Winogradsky’s solutions were the nitrogen compounds, and it consequently followed that the organisms must derive their energy supply by the oxidation of ammonia and nitrite respectively. The release of energy obtained by these two reactions has been calculated by Orla-Jensen to be as follows:—
(NH4)2CO3 + 3O2 = 2HNO2 + CO2 + 3H2O + 148 Cals.
KNO2 + O = KNO3 + 22 Cals.
The exact process by which ammonium carbonate is converted into nitrite is not at present known. The two groups of organisms are extremely selective in their source of energy. The nitrous organisms can derive their energy only by the oxidation of ammonia to nitrite, and the nitric[36] organisms only by the oxidation of nitrite to nitrate. In culture media they are, indeed, inhibited by soluble organic compounds such as sugars. Under natural conditions, however, they appear to be less sensitive, since ammonium carbonate is readily nitrified in substrata rich in organic matter. The rapid nitrification that takes place during the purification of sewage is an example of this. The conditions in culture, with regard to aeration and the removal of metabolic products from the neighbourhood of the organisms, are very different from those in the soil, and perhaps account for the discrepancies found.
The oxidation of ammonium carbonate by nitrosomonas results in the formation of nitrous acid. The organisms are very sensitive to acidity, and can only operate if the nitrous acid produced is neutralised by an available base. In normal soils calcium carbonate supplies this base, and in acid soils the formation of nitrite is, as a rule, increased by the addition of lime, or of calcium or magnesium carbonate. There is evidence that in the absence of calcium carbonate, other compounds can be used as a base. It was found by Hopkins and Whiting[32] that in culture solution the nitrifying organisms could use insoluble rock phosphate as a base, producing therefrom the soluble acid phosphate. There is evidence, however, that in ordinary soil containing calcium carbonate very little solution of phosphate takes place in this way. The further oxidation of nitrite to nitrate by Nitrobacter does not produce acid, and requires no further neutralising base.
The nitrate produced in this way is the main source of nitrogen supply to plants under normal conditions. Experiments have shown that a number of plants are capable of utilising ammonia as a source of nitrogen, and Hesselmann[34] has found forest soils in Sweden where no nitrification was proceeding, and where, therefore, plants would presumably obtain their nitrogen in this way, but such cases must be regarded as exceptional.
Another group of bacteria capable of deriving their energy[37] from an inorganic source exists in the soil. This comprises the sulphur bacteria, which are able to derive energy by the oxidation of sulphur, sulphides, or thiosulphates to sulphuric acid:—
S + 3O + H2O = H2SO4 + 141 Cals.
One organism studied by Waksman and Joffe[63] is able to live in inorganic solution, deriving its carbon from carbon dioxide. The sulphur bacteria have recently come into prominence in America owing to their faculty for producing acid. Thus Thiospirillum will increase the acidity of its medium to a reaction of PH 1·0 before growth ceases. The potato scab disease in America is now treated by composting with sulphur. This treatment depends on the production of sulphuric acid by the sulphur oxidising bacteria, which renders the soil too acid for the parasite. There is some evidence also that acid thus produced can be used to render insoluble phosphatic manures more available in the soil.
Analogous to the sulphur organisms are certain bacteria isolated from sheep dig tanks in South Africa by Green,[28b] which can derive energy by the oxidation of sodium arsenite to arsenate.
As is seen in the examples mentioned, energy is commonly obtained by bacteria through an oxidation process in which free oxygen is utilised. In water-logged soil, however, or in soil overloaded with organic matter, anaerobic bacteria may develop, which obtain their oxygen from oxidised compounds. Thus there are soil organisms described by Beijerinck[2] and others which can obtain oxygen by reducing sulphates to sulphides.
A more important source of oxygen under these conditions is nitrate, which can supply oxygen to a larger number of bacteria. The stage to which the reduction can be carried varies according to the organism. A very large number of bacteria are capable of reducing nitrates to nitrites. Many can reduce nitrate to ammonia, and some[38] can produce an evolution of nitrogen gas from nitrate. The effects of nitrate reduction, therefore, appear under water-logged conditions in soils. For example, in swamp soils in which rice is grown, it has been found by Nagaoka,[53] in Japan, that treatment with nitrate of soda depresses the yield, probably owing to the formation of poisonous nitrites by reduction.
Under normal conditions of well aerated soil, however, it is unlikely that the reduction of nitrate is of great importance. In such soils the activities through which bacteria acquire their energy are, as we have seen, of vital importance to the plant, resulting in the disintegration of plant tissues, with the ultimate formation of humus, and in the production of nitrate.
In their activities connected with the building up of their protoplasm, bacteria may, on the other hand, compete with the plant. These activities and their consequences will be reviewed in the following chapter.
[39]
The activities of the soil bacteria that we have yet to consider are those connected with the building-up from simpler materials of the protoplasm of the bacterial cell. It is important to bear in mind that this process is one requiring an expenditure of energy on the part of the organism. The sources of energy we have already considered.
The bodies of bacteria contain the same elements common to other living matter. Analyses of various bacteria have been made by a number of workers. About 85 per cent. of their weight is made up of water. This analysis of Pfeiffer’s Bacillus by Cramer[15] shows the typical percentages of carbon, nitrogen, hydrogen, and ash in the dry matter:—
Composition of Pfeiffer’s Bacillus (Cramer).
| C | 50 | per cent. |
| N | 12·3 | „ |
| H | 6·6 | „ |
| Ash | 9·1 | „ |
About 65-70 per cent. of the dry matter of bacteria consists of protein.
The biggest constituent of the dry matter of bacteria is therefore carbon. In the soil, bacteria find an abundance of[40] organic matter from which they may derive their carbon supply. A special case, however, is furnished by the nitrifying organisms, certain sulphur oxidising bacteria, and others that derive their carbon from the CO2 of the soil atmosphere. The sources from which these special groups obtain the necessary energy to accomplish this, we have already considered.
Of chief importance in its consequences are the means adopted by bacteria to obtain their nitrogen supply.
There is some reason to believe that soil bacteria do not take up protein and peptones as such, but must first break down these bodies into simpler compounds. When a sufficient amount of easily decomposable organic nitrogen is present in the soil, the ammonifying bacteria use such compounds as sources of energy, and in this case have a nitrogen supply exceeding their requirements.
But where there is an excess of carbohydrate or other non-nitrogenous source of energy available in the soil, the case is different. Here the organisms have a supply of energy which enables them to multiply rapidly until the organic nitrogen is insufficient for their needs. Hence they turn to the ammonia and nitrate present in the soil, and build up their proteins from this source. Doryland[17] has shown that many common soil ammonifiers assimilate ammonia and nitrate when supplied with carbohydrate. There may thus be a temporary loss of nitrate from soil when sugar, starch, straw, or such materials are added to it.
The bacteria that we have so far considered take up their nitrogen directly from compounds containing this element. There remain, however, a comparatively small but very important group of bacteria possessing the power of causing elemental nitrogen to combine, and of building it up into[41] their proteins. This fixation of nitrogen by micro-organisms is a vital step in the economy of nature. Losses of nitrogen from the land are continually occurring through the washing-out of nitrates by rain, and through the evolution of gaseous nitrogen during the processes of decay. To maintain the supply of combined nitrogen which is essential to living organisms, there must therefore be a compensating process by which the supply of nitrogen compounds in the soil is kept up.
It was discovered in the middle of the nineteenth century that if soil were kept moist and exposed to the air, there was an increase in the amount of nitrogen compounds present. Berthelot, in 1893, studied the nitrogen relationships of soil, and recognised that this fixation of nitrogen in soil was the work of micro-organisms.
Winogradsky followed up his work and isolated from soil a large anaerobic spore-forming organism, capable of fixing nitrogen, to which he gave the name Clostridium pasteurianum. In 1901 the investigations of Beyerinck, in Holland, led to the important discovery of a group of large aerobic organisms, which he named Azotobacter. These were found to be very active in fixing free nitrogen. More recently, a number of other nitrogen-fixing bacteria have been described, and the property has been found to exist to a small extent in several previously well-known organisms.
It becomes important to determine which are the groups of bacteria whose nitrogen-fixing powers are of chief importance in the soil.
On account of its energetic fixation of nitrogen in culture media, Azotobacter has attracted the greatest attention of workers. The evidence seems to be consistent with the view that Azotobacter is of importance in the soil. Thus the distribution of Azotobacter would appear to be world-wide. It is found all over Western Europe and the United States. Lipman and Burgess[45] found it in soils collected from Italy and Spain, Smyrna, Cairo, the Fayum, the Deccan in India, Tahiti, Hawaii, Mexico, Guatemala, and Canada. C. M.[42] Hutchinson[29] found it to be distributed throughout India. It was found by Omelianski[55] to be widely distributed in European and Asiatic Russia, and by Groenewege[28] in Java. Ashby[1] at Rothamsted, isolated it from soils from the Transvaal, East Africa, and Egypt. Also, an association has sometimes been found between the ability of a soil to fix nitrogen and the occurrence and vigour of its Azotobacter flora. Thus Lipman and Waynick[46] found that if soil from Kansas were removed to California, its power to produce a growth of Azotobacter, when inoculated into a suitable medium, was lost, and, at the same time, its nitrogen-fixing power was greatly reduced. Moreover, it is known that conditions favourable to the fixation of nitrogen by Azotobacter in cultures on the whole favour nitrogen fixation in soils. The conditions that favour other aerobic nitrogen-fixing bacteria are, however, not sufficiently distinct to make such evidence of great value.
It is usually found that nitrogen fixation is most active in well-aerated soil. Thus Ashby,[1] at Rothamsted, found the nitrogen-fixing power of a soil to decrease rapidly with depth. Similar results were obtained in Utah by Greaves. This suggests, at first sight, that anaerobic nitrogen fixers are unimportant under normal soil conditions. It is, however, quite possible that they may assume an importance when acting in conjunction with aerobic organisms. Thus Omelianski and Salunskov[55] found that beneficial association, or symbiosis, could occur between Azotobacter and Clostridium pasteurianum, the former absorbing oxygen from the surroundings, and thus creating a suitable anaerobic environment for the Clostridium.
The question of symbiosis of nitrogen-fixing bacteria with each other and with other organisms offers an inviting field for research. There is evidence that this factor may have considerable importance. Beijerinck and Van Delden[3] early recognised that Azotobacter in mixed cultures fixed more nitrogen than in pure cultures. Granulobacter, an organism which they found to be commonly associated with Azotobacter[43] in crude cultures, appears to increase its nitrogen-fixing powers (Krzeminiewski).[41] It was also found by Hanzawa[31] that a greater fixation of nitrogen was obtained when two strains of Azotobacter were grown together. A symbiosis between Azotobacter and green algæ has been described, and will be further discussed by Dr. Bristol. It is likely that this association may be of importance under suitable conditions on the soil surface where the algæ are exposed to light.
The combination of elemental nitrogen is an endothermic process which requires a very considerable amount of energy for its accomplishment. This fact is well illustrated by the various commercial processes in use for fixation of atmospheric nitrogen. The nitrogen-fixing bacteria obtain this energy from the carbon compounds in the soil. A number of compounds were compared as sources of energy by Löhnis and Pillai,[47] who tested their effect on the amounts of nitrogen fixed by Azotobacter in culture. It was found that mannitol and the simpler sugars give the best results as sources of energy, but that other organic compounds can also be used. Mockeridge[51] has adduced evidence that ethylene glycol, methyl-, ethyl-, and propyl-alcohol, lactic, malic, succinic, and glycocollic acids could also be utilised. Since so large a part of the organic matter added to soil is in the form of celluloses, it is of great importance to ascertain how far these compounds and their decomposition products can be utilised in nitrogen fixation. Stubble, corn-stalks and roots, oak leaves, lupine and lucerne tops, maple leaves, and pine needles may all serve as useful sources of energy to nitrogen-fixing organisms in the soil. Pure cellulose cannot apparently be used as a source of energy, but when acted upon by cellulose decomposing organisms, it becomes available as a source of energy. Hutchinson and Clayton, at Rothamsted, found that a fixation of nitrogen could be brought about by mixed cultures of Azotobacter, and of the cellulose attacking Spirochæta cytophaga, when grown in cultures containing pure cellulose. It is not known how far cellulose decomposition must proceed to produce an effective source of energy,[44] nor what are the substances thus produced that are utilised. This point will not be decided until something more is known of the course of changes in the breaking-down of cellulose in the soil.
The amount of nitrogen fixed per unit of energy material decomposed varies greatly, according to the organism and the conditions. Winogradsky found that his Clostridium assimilated 2-3 mgs. of nitrogen per gram of sugar consumed. Lipman found that Azotobacter fixed 15-20 mgs. of nitrogen per gram of mannite consumed.

Fig. 5.
Caption: Azotobacter. Decrease in efficiency in N fixation with age of culture. (Koch & Seydel.)
X-axis: Days.
Y-axis: Milligrams of Nitrogen fixed per gram of dextrose consumed.
It is found, however, that in liquid culture, the ratio of nitrogen fixed to carbohydrates oxidised varies according to the age of the culture, falling off rapidly as the age increases[42] (Fig. 5). This decreasing efficiency in cultures may be due to the accumulation of metabolic products such as would not occur under soil conditions. Indeed, the efficiency of Azotobacter in a sand culture has been found by Krainskii[39] to be considerably greater than in solution. It is thus probable that in soil the nitrogen-fixing organisms are less[45] wasteful of energy material than under the usual laboratory conditions. It is to be hoped that future research will indicate what are the conditions that produce the greatest economy of energy material in nitrogen fixation.
The fixation of nitrogen in soil is depressed by the presence of considerable amounts of nitrates. This is, in all probability, due to the fact that nitrogen-fixing organisms are able to utilise compounds of nitrogen where these are available. The energy needed to build up amino-acids and proteins from nitrate or ammonia is, of course, far less than that required to build up these substances from elemental nitrogen. It is, therefore, not surprising that where nitrate is available, Azotobacter will use it in preference to fixing atmospheric nitrogen.[5]
TABLE III.—ASSIMILATION OF NITRATES.
By Azotobacter in Pure Culture—(Bonazzi).
| Nitrate and Nitrite Present. |
Organic Nitrogen and Ammonia Present. |
Total Fixed or Lost. |
|
|---|---|---|---|
| mgs. | mgs. | mgs. | |
| Culture with nitrate— | |||
| At beginning | 8·55 | 0·76 | — |
| After growth | 0·2 | 8·71 | - 0·4 |
| Culture without nitrate— | |||
| At beginning | — | 0·76 | — |
| After growth | — | 4·50 | + 3.74 |
| (Growth period—24 days at 25° C.) | |||
The chemical process by which nitrogen is fixed is quite unknown, although a number of speculative suggestions have been made. The appearance of considerable amounts of amino acids in young cultures of Azotobacter suggests that these may be a step in the process, but at present the data are too inconclusive to form a basis for theorising.
Azotobacter is very rich in phosphorus, an analysis of the surface growth in Azotobacter cultures, made by Stoklasa, giving about 60 per cent. of phosphoric acid in the ash. In cultures it has been found that a considerable amount of[46] phosphate is needed to produce full development. As would be expected, therefore, nitrogen fixation in soil is often greatly stimulated by the addition of phosphates. Christensen has, indeed, found soils where lack of phosphate was the limiting factor for Azotobacter growth.
Azotobacter is very intolerant of an acid medium, and is very dependent on the presence of an available base. In cultures this is usually provided in the form of calcium or magnesium carbonate. Gainey[21] found that Azotobacter occurred in soils having an acidity not greater than PH 6·0, and Christensen,[7], [9] in Denmark, has found a close association between the occurrence of Azotobacter in soils and the presence of an adequate supply of calcium carbonate. So close was this association that he devised a technique based on this fact for detecting a deficiency of lime in a soil sample.
In addition to the groups already discussed, there is a remarkable and important group of nitrogen-fixing bacteria that inhabit and can carry on their functions within the root tissues of higher plants. It has been known at least from classical times that certain leguminous plants would, under suitable conditions, render the soil more productive. On the roots of leguminosæ small tubercles are commonly found. These were noted and figured by Malpighi in the seventeenth century, and for a long time were regarded as root-galls. As was described in Chapter I., the true nature of these tubercles was finally elucidated by Hellriegel and Wilfarth in 1886. As the result of a series of pot experiments, they made the very brilliant deduction that the ability to fix nitrogen, possessed by the legumes, was due to bacteria associated with them in the tubercles.
These bacteria were finally isolated and studied in pure culture by Beijerinck. Since then a very great deal of literature has accumulated on the subject of the nodule-producing bacteria, which it is impossible to deal with in a small space. The nodule organism, Bacillus radicicola, when grown on suitable media, passes through a number of different changes in morphology. The most connected account of[47] these changes is given in a paper by Bewley and Hutchinson.[4] In a vigorous culture the commonest type is a rod-shaped bacillus which may or may not be motile. As these get older they often become branched, or irregular in shape, the formation of these branched forms being perhaps due to conditions in the medium. These irregular forms, known as “bacteroids,” are a characteristic type in the nodules. Their production in culture media has been found to be stimulated by sugars and organic acids such as would occur in their environment within the host plant. In the older rods and bacteroids the staining material becomes condensed into granules, and finally the rods disintegrate or break up into coccoid forms. By suitable culture conditions, Bewley and Hutchinson obtained cultures consisting almost entirely of this stage. If such a culture be inoculated into a fresh medium rich in sugar, the swarmer stage appears in great numbers. These swarmers are very minute coccoid rods, ·9 × ·18 in size, that are actively motile. They apparently develop later into the rod stage.
Fig. 6.—Bacillus radicicola. Stages in the life cycle. (After Hutchinson and Bewley.)
Motile Rods
Vacuolated Stage
“Swarmers”
“Bacteroids”
“Pre-swarmers”
Very little is known as to the life of the organism in the soil. It is able, however, to fix nitrogen in cultures, and it[48] has been claimed[35], [48] that it can do so in the soil outside the plant, so that it is possible that we must take it into consideration in this connection. More knowledge is needed as to the optimum conditions for the growth of the organism in the soil. It seems to be more tolerant of acid soil conditions than Azotobacter. The limiting degree of acidity has been found to vary among different varieties of the organism from PH 3·15 to PH 4·9.
A long controversy has been held as to whether the nodule organisms found in different host-plants all belong to one species, or whether there are a number of separate species, each capable of infecting a small group of host-plants. As the term “species” has at present no exact meaning when applied to bacteria, the discussion in this form is unlikely to reach a conclusion. The evidence seems to show that the nodule organisms form a group that is in a state of divergent specialisation to life in different host-plants, and that this specialisation has reached different degrees with different hosts. Thus the organisms from the nodule of the pea (Pisum sativum) will also produce nodules on vicia, Lathyrus, and Lens, but seem to have lost the ability normally to infect other legumes. On the other hand, the bacteria from the nodules of the Soy Bean (Glycine hispida) have become so specialised that they do not infect any other genus of host-plant, and soy beans are resistant to infection by other varieties of the nodule organism. Burrill and Hansen,[6] after an extensive study, divided the nodule bacteria into eleven groups, within each of which the host-plants are interchangeable. The existence of different groups of nodule organisms has been confirmed by the separate evidence of serological tests (Zipfel, Klimmer, and Kruger).[40] The results of cross-inoculation tests have sometimes been conflicting. It seems, indeed, that the host-plant has a variable power of resisting infection, so that when its resistance is lowered it may be capable of infection by a strange variety of the nodule organism. The question that has thus arisen of the ability of the legume to resist infection[49] is of fundamental importance, and its elucidation should throw light on the relation of plants to bacterial infection as a whole.
The stage of the organism that infects the plant is not at present known. It may be supposed that it is the motile “swarmer.” The entry is normally effected through the root-hairs. The hair is attacked close to the tip, and an enzyme is apparently produced which causes the tip to bend over in a characteristic manner. The organisms multiply within the root hair and pass down it, producing a characteristic gelatinous thread filled with bacteria, in the rod form. This “infection thread” passes down into the cells of the root tissue, where it branches profusely. In young stages of nodule formation the branches can be seen penetrating cells in the pericycle layer. Rapid cell division of these root cells is induced. In the course of this cell division abnormal mitotic figures are sometimes found, such as occur in pathological growths. The cells push outward the root cortical layer, and so form a nodule.
Certain of the cells in the centre of the nodule become greatly enlarged, and in the fully grown nodule are seen to be filled with bacteria. Differences have been described in the morphology of the organisms in different parts of the nodule.[62] Whether the different stages of the organism are equally capable of fixing nitrogen, or what is the significance of these stages within the nodule, is not certainly known. It has been held that it is the irregular bacteroid forms that are chiefly concerned with nitrogen fixation. In older nodules the organisms become irregular and stain faintly, and the bacteroidal tissue breaks down, the nodule finally decaying. In the fixation of nitrogen that occurs in the nodules, the bacteria without doubt derive the necessary energy from the carbohydrates of the host-plant. There is evidence that the plant assists the process of fixation by removing soluble metabolic products from the neighbourhood of the bacteria. Golding[22] was able to obtain a greatly increased fixation of nitrogen in artificial cultures[50] by arranging a filtering device so as to remove the products of metabolism.
The great practical importance of leguminous crops in agriculture has led to numerous attempts being made to increase their growth, and the fixation of nitrogen in them, by inoculating the seed or the soil with suitable nodule-bacteria. This inoculation can be effected either with soil in which the host-plant has been successfully grown, and which should consequently contain the organism in fair numbers, or else pure cultures of the organisms isolated from nodules may be used. Very varying results have been obtained with inoculation trials.
In farm practice a leguminous crop has often been introduced into a new area where it has never previously grown. In such soil it is very probable that varieties of the nodule organism capable of infecting the roots may not exist. In such cases inoculation with the right organism or with infected soil often produces good results.
The more difficult case, however, is that in which the legume crop has been grown for a long time in the locality, and where the soil is already infected with right organisms. This, the more fundamental problem, applies especially to this country. Here it would seem that inoculation with a culture of the organism will benefit the plant only (1) if the naturally occurring organisms are present in very small numbers; or (2) if the organisms in the culture added are more virulent than those already in the soil. The problem of successful inoculation would therefore seem to be bound up with that of grading up the infective virulence of the organism to a higher level.
Successful nodule development in a legume crop is also dependent to a large degree on the soil conditions. The effects of soil conditions on nodule development have been studied by numerous workers. Moisture has been found very greatly to affect the nodule development. Certain salts have a very definite effect on nodule formation.[64] Their effect on the number of nodules developing has been studied,[51] but the reason for this effect is unusually difficult to decide. The action is usually a complex one. Thus phosphates are known to stimulate nodule formation. They probably act in several ways. In the first place, they may cause the nodule organisms to multiply in the soil; in the second place, they produce a greater root development in the plant, thus increasing the chances of infection; and in the third place, Bewley and Hutchinson[4] have found that phosphates cause the appearance of the motile stage of the organism in cultures. A real understanding of the influence of environment on nodule production will produce great improvements in our methods of legume cropping.
The various activities of the soil bacteria have a vital importance to the growth of higher plants, which are dependent for their existence on certain of these processes. In the first place, as we have seen, bacteria decompose the tissues of higher plants and produce humus materials, which are essential to the maintenance of good physical properties in the soil. Then the nitrate supply on which most higher plants depend is produced by the decomposition of organic nitrogen compounds by bacteria in their search for energy. The depletion of the total nitrogen content of the soil through rain and through the removal of nitrogen in the crops, is to some extent compensated by the fixation of atmospheric nitrogen by certain bacteria. On the other hand, in the assimilation of nitrogen compounds to build up protein, the bacteria are competing with higher plants for one of their essential food constituents, and their action may, under certain conditions, cause a temporary nitrogen starvation. One must remember, however, that large quantities of nitrate are lost from field soils by washing-out through rain action, especially in winter. The assimilation of nitrate and ammonia by micro-organisms keeps some of this nitrogen in the soil, and at certain periods may thus be beneficial.
[52]
There is another important respect in which soil bacteria influence plant growth. Their activities result in the release of inorganic salts, such as potash and phosphates, in a form available for the use of plants. The release of phosphorus and potassium compounds takes place in two ways. In the first place, organic matter containing phosphorus and potassium, in an insoluble form, is attacked by bacteria, resulting in these elements being set free as inorganic salts available to the higher plant. Secondly, much of the phosphorus supplied to the soil from rock minerals is present as insoluble phosphates, such as apatite and iron phosphate. Much of the potassium, too, is derived from insoluble silicate minerals. In both cases the conversion of the insoluble minerals into soluble phosphates and potassium compounds is brought about to a large extent by solution in water containing carbonic and other acids. These acids are largely produced by micro-organisms, which, in addition to carbonic acid, produce organic acids, and in specialised cases, sulphuric and nitrous acids. It has been found, for example, that in a compost of soil with sulphur and insoluble phosphate, sufficient sulphuric acid may be produced by the oxidation of the sulphur by bacteria to convert an appreciable amount of phosphate into a soluble form. When we consider the functions performed by soil bacteria, therefore, it is not surprising to find that high bacterial activity in the soil is associated, as a rule, with fertility.
The object of soil bacteriologists is to discover means of favouring the activity of soil bacteria, especially those activities that are useful to the higher plant. Knowledge is therefore needed of the changes in numbers and activities of the soil bacteria, and of the influence of soil conditions on them. The necessity of studying these changes has required the development of a quantitative technique by which the[53] numbers of bacteria in the soil and their activities can be estimated.
The method commonly used in counting bacteria in soil is a modification of the plating method of Koch. In counting bacteria two difficulties have to be overcome—their immense numbers and their small size. The numbers of bacteria in soil are so large that the bacterial population of a gram of soil could not, of course, be counted directly. The method adopted, therefore, is to make a suspension of soil in sterile salt solution, and to dilute this suspension to a convenient and known extent, which will depend on the numbers of bacteria expected. In ordinary field soils it is found convenient, for example, to dilute the soil suspension so that one cubic centimeter of the diluted suspension will contain 1⁄250,000th of a gram of soil. Such a volume will commonly contain a number of bacteria sufficiently small to count. The second difficulty is that the organisms are microscopic, and yet cannot be readily counted under the microscope owing to the presence of soil particles in the suspension. Hence recourse is had to plating. One cubic centimeter of diluted suspension is placed in a petri dish and mixed with a suitable nutrient agar medium, melted, and cooled to about 40° C. The medium sets, and after a few days’ incubation the organisms multiply and produce colonies visible to the naked eye. By counting these colonies we obtain an estimate of the number of bacteria in the one cubic centimeter of suspension, it being assumed that every organism has developed into one colony, and by multiplying this number by the degree of dilution we obtain the numbers per gram of soil. In practice a number of parallel platings are made from one cubic centimeter portions of the diluted suspension and the mean number of colonies per plate is taken. By this means the error due to the random distribution of bacteria in the suspension is reduced, because of the greater number of organisms counted.
In drawing conclusions from bacterial count data, it is necessary to distinguish between the indication which the[54] method gives of the absolute numbers of bacteria in the soil and the accuracy with which it enables the numbers of two soil samples to be compared. The method cannot be used for the former purpose at present. We do not know how far the figures obtained by this counting method fall short of the actual number of bacteria in the soil. One reason for this is the difficulty of effecting a complete separation of the clumps of bacteria into discrete individuals in the suspension. Then again, there is no known medium upon which all the physiological groups of bacteria will develop and produce colonies. And even on a suitable medium some of the individuals may fail to multiply.
In comparing the bacterial numbers in two soil samples, however, the case is different. Within each bacterial group investigated the plate method should give counts proportional to the bacterial numbers in the soil. Thus, by the method one should be able to tell whether the bacterial numbers are increasing or decreasing over a period of time, or whether a certain soil treatment produces an increase or a decrease. With this end in view the technique of the method has been improved by recent workers. It was found that, when carefully standardised, the process of dilution of the soil could be carried out without significant variation in result (Table IV.), and that the accuracy of the method is limited mainly by the variation in colony numbers on parallel platings, due in part to random distribution of bacteria throughout the final suspension, and partly to the uneven development of colonies on the medium. The question of the medium was therefore taken up with a view to improving the uniformity of results obtained with it. Lipman, Conn, and others effected an improvement by using chemical compounds as nutrient ingredients, thus making their media more closely reproducible. On most agar media, an important disturbing factor is the growth of spreading colonies, which prevent the development of some of the other colonies. A medium has been devised at Rothamsted on which these spreading organisms are largely restricted.[61][55] A statistical examination[19] has shown that on this medium errors due to the uneven development of colonies, except in special cases, are prevented, so that in fact the variation in colony numbers between parallel plates is found to be that produced merely by random distribution of bacteria in the diluted suspension (see Table IV.). In this case the accuracy of the counts of the bacteria in the diluted suspension depend directly on the number of colonies counted, and can be known with precision.
TABLE IV.—BACTERIAL COUNTS OF A SOIL SAMPLE.
Parallel Plate Counts from Four Sets of Dilutions made by
Different Workers.
| Counts of Colonies on each Plate. | ||||
|---|---|---|---|---|
| Plate. | Set I. | Set II. | Set III. | Set IV. |
| 1 | 72 | 74 | 78 | 69 |
| 2 | 69 | 72 | 74 | 67 |
| 3 | 63 | 70 | 70 | 66 |
| 4 | 59 | 69 | 58 | 64 |
| 5 | 59 | 66 | 58 | 62 |
| 6 | 53 | 58 | 56 | 58 |
| 7 | 51 | 52 | 56 | 54 |
| Mean | 60·86 | 65·86 | 64·28 | 62·86 |
| Standard deviation between the four sets = 5·62. | ||||
| Standard deviation between plates within the sets = 7·76. | ||||
The knowledge obtained from counts of soil bacteria is subject to another serious limitation. We do not know which of the bacteria counted are the most effective in bringing about the various changes that take place in the soil. It is not even known which of them are active in the soil and which are in a resting condition. It is thus possible to have two soils containing equal numbers of bacteria but showing widely different biochemical activity, if one soil contains organisms of a higher efficiency. Moreover, as has been pointed out, many important groups of soil bacteria do not develop on the plating media, and so are not counted. These considerations led to the development of supplementary[56] methods by which it was hoped to estimate the actual biochemical activity of the soil microflora. The first of these methods was developed by Remy, who attempted to study the biochemical activity of a soil by placing weighed amounts into sterile solutions of suitable and known composition, keeping them under standard conditions for a definite time and then estimating the amount of the chemical change that was being studied. Thus, to test the activity of the organisms that produce ammonia from organic nitrogen compounds, he inoculated soil into 1 per cent. peptone solution and measured the amount of ammonia produced in a given time. By similar methods the power of a soil to oxidise ammonia to nitrate, to reduce nitrate, or to fix atmospheric nitrogen, is tested. This method has been extensively used and developed by more recent workers. It suffers, however, from the same serious disadvantage that it was designed to avoid, for we cannot be certain that those bacteria that develop in the nutrient solution are the types that are active in the soil, and, moreover, even where the same types do function in the two conditions, we do not know that the degree of their activity is the same in soil and in solution cultures. For instance, Nitrosomonas appears to show very different degrees of activity in soil and in culture.
Another method, therefore, of studying the activity of soil micro-organisms is the obvious one of estimating the chemical changes that they produce in the soil itself. This method has obvious advantages over the unnatural methods developed from Remy’s, but it has a number of limitations that make its actual application difficult. In the first place, we cannot always tell whether changes found to occur in soil are due to the activity of micro-organisms, or are purely chemical reactions unassisted by biological agencies. Then, if we succeed in showing that the changes are due to micro-organisms, it is very difficult to determine which organisms are effecting them. This cannot be definitely tested by isolating suspected organisms and testing their activity in sterile soil, because in sterilising soil its nature and composition[57] is altered. In spite of these difficulties, however, the study of the chemical changes that take place in the soil has produced valuable knowledge, when it has been combined with a study of the changes in the number and variety of the micro-organisms that accompany these reactions. This method of investigation is well illustrated by the work of Russell and Hutchinson on the effects of heat and volatile antiseptics on soil, where a study of the chemical changes such as ammonia production, that occurred in these treated soils, combined with a study of the changes in bacterial numbers, led to the realisation that the soil micro-population was a complex one, containing active protozoa.
A great difficulty in applying quantitative methods to bacteria in the field is the great variation in the density of the bacterial population over a plot of field soil, which may be so great that a bacterial count from a single sample is quite valueless. For example, the distribution of bacterial numbers over a plot of arable soil near Northampton was studied by taking sixteen samples distributed over an area about 12 feet square. The result showed that in some cases the bacterial numbers in samples taken 6 inches apart differed by nearly 100 per cent. Fortunately, under favourable conditions, a remarkably uniform distribution of bacterial numbers over a plot of soil can be found.
On such a plot it is possible to investigate the rapidity with which the numbers of the soil micro-organisms alter in point of time. For example, on the dunged plot of Barnfield, Rothamsted, which has been cropped with mangolds for forty-seven successive years, the area distribution of bacteria has been found to be so uniform that if a number of samples of soil are taken from the plot at the same time, the difference in bacterial numbers between the samples cannot be detected by means of the counting technique (see Table V.). The work of Cutler, Crump, and Sandon[16] on this plot showed that the bacterial numbers vary very greatly from one day to the next, and that these fluctuations took place over the whole plot, since two series of samples, taken[58] in two rows 6 feet apart, showed similar fluctuations (see Fig. 7). The discovery of these big daily fluctuations in numbers led to an inquiry as to how quickly bacterial numbers change, and samples from Barnfield, taken at two-hourly intervals, showed that significant changes in numbers took place even at such short intervals.
TABLE V.—BACTERIAL COUNTS OF FOUR SOIL SAMPLES.
From Barnfield, Taken Simultaneously.
| Counts of Colonies on each Plate. | ||||
|---|---|---|---|---|
| Plate. | Sample I. |
Sample II. |
Sample III. |
Sample IV. |
| 1 | 38 | 45 | 43 | 27 |
| 2 | 32 | 40 | 34 | 41 |
| 3 | 52 | 45 | 52 | 35 |
| 4 | 32 | 31 | 55 | 36 |
| 5 | 40 | 43 | 38 | 45 |
| Mean | 38·8 | 40·8 | 44·4 | 36·8 |
| Standard deviation between the four samples = 7·25. | ||||
| Standard deviation between parallel plates within the sets = 7·55. | ||||
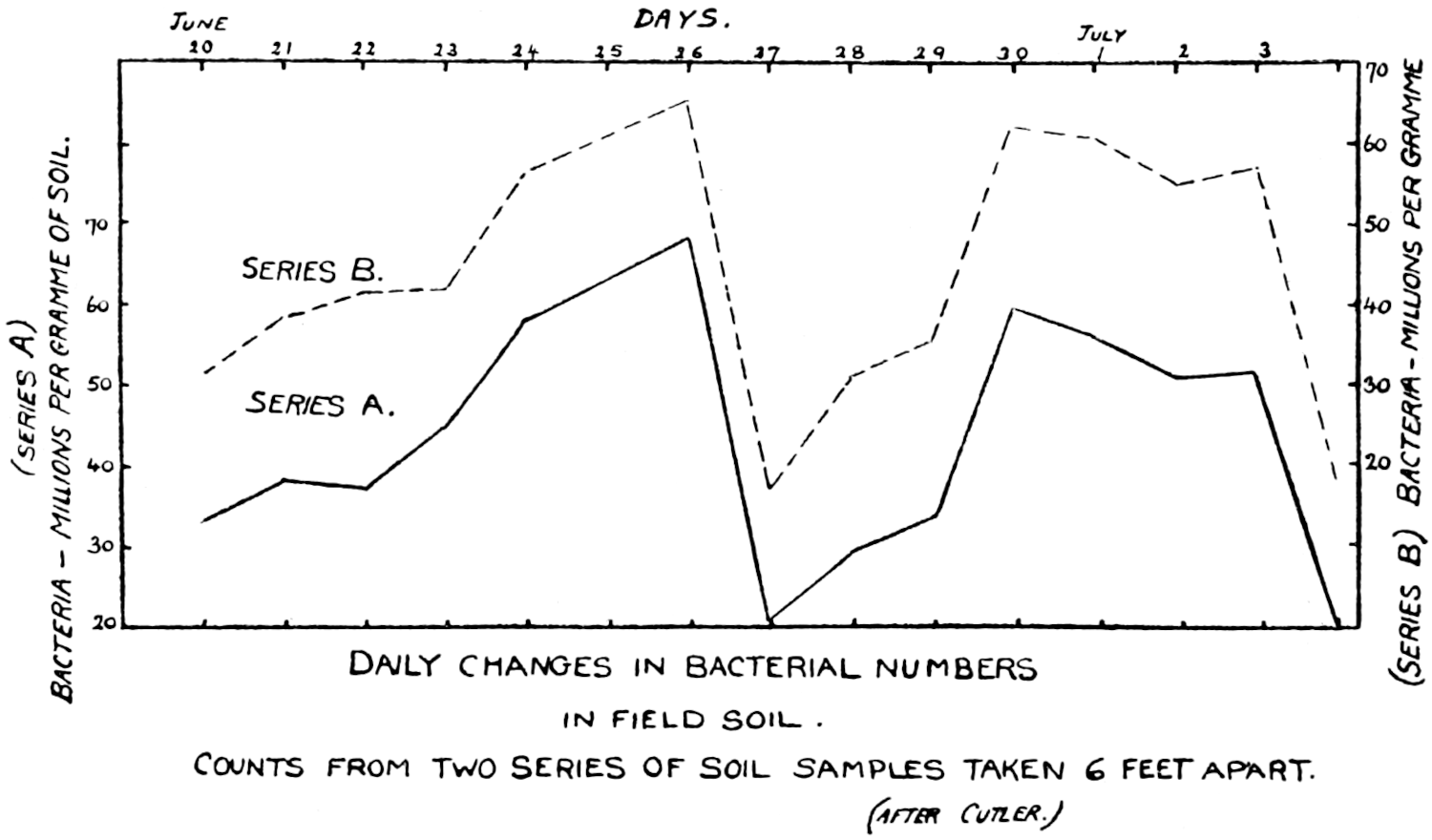
Fig. 7.
X-axis (top): Days.
Y-axis (left): (Series A) Bacteria—millions per gramme of soil.
Y-axis (right): (Series B) Bacteria—millions per gramme.
Caption: Daily changes in bacterial numbers in field soil.
Counts from two series of soil samples taken 6 feet apart.
(After Cutler.)
Since the bacteria involved in this fluctuation are of great importance to the crops, being for the most part[59] ammonia producing types, further knowledge as to the cause of this fluctuation and of its effect on the ammonia and nitrate in the soil is of fundamental importance. There is evidence, which will be discussed later, that the cause is connected with the changing activities of certain soil protozoa, since the daily changes in the numbers of active amœbæ in the soil have been found to be in the reverse direction to those of the bacterial numbers. It appears, therefore, that we are dealing with an equilibrium between the various members of the soil population, the point of equilibrium changing at frequent intervals.
In addition to daily changes, it is possible to detect changes in the numbers and activity of the soil population related to the season. There is a well-marked increase in the spring and autumn (see Figs. 15, 16, pp. 89, 90). This is well seen when the fortnightly averages of the daily bacterial and protozoal counts from Barnfield soil are plotted. These spring and autumn increases comprise both the bacterial and the protozoal population, and therefore differ from the short time fluctuations in being due, not to a disturbance of the bacteria-protozoa equilibrium, but to a general rise in activity of both groups of organisms.
When we consider the action of external conditions on the soil bacteria, the existence of a complex soil population and the interdependence of its members must be borne in mind. Changes in external conditions may affect the different components of the population in different ways or to different degrees, thus upsetting the equilibrium between the various groups. For example, the addition of a mild aromatic antiseptic to the soil apparently affects the protozoa in such a way as to disturb the bacteria-protozoa equilibrium in favour of the bacteria, while in some cases the aromatic compound affords a food supply to special bacteria, causing these to increase, upsetting the equilibrium between the different bacterial groups. When our knowledge of the effect of external factors on the soil population becomes sufficient, it will probably be found that in nearly all cases[60] a change in the soil conditions produces some disturbance in the equilibrium between the components of the soil population, though at present there are only certain examples where this disturbance is a probable explanation of the facts.
Since bacteria are dependent on adequate supplies of energy and food, it is to be expected that additions of organic matter or of inorganic food materials will greatly benefit their activities. The effect of added farmyard manure in increasing bacterial activities has been much studied.[27] Some of the increased bacterial numbers and activities in this case may be due to the addition of bacteria with the manure, but it is thought that this factor is of less importance than the added energy and food supply which the general soil flora obtain from it. Nutritive salts such as phosphates and salts of potassium usually increase the bacterial activities.
The effect of alkali salts on soil bacteria has been especially studied in the Western United States, where the existence of alkali in the soil is a serious problem.[23] Soil bacteria are usually stimulated by small doses of alkali salts that are toxic in higher concentration. As a rule, chlorides are the most toxic salts, the electronegative ion playing an important part in the effect of the salt. Salts affect bacteria both owing to the changes in osmotic pressure which they produce, and through their specific action on the bacterial protoplasm.[26] When equal weights of various salts are added to soil, their toxic action on bacteria shows so little association with their respective osmotic pressures that we must conclude that this factor is the less important. There is reason to suppose that the toxic action of salts on bacteria is often connected with an effect of the specific ions on the permeability of the bacterial cell-wall. This conclusion is based on the changes in electrical conductivity of bacterial suspensions in the presence of various salts.[59]
A definite antagonism between various salts has been found to exist. It is possible that future work in this line may indicate what are the proportions of common electrolytes[61] which will produce a properly “balanced” soil solution so that the harmful excess of one salt may be antagonised.
Certain salts, such as those of arsenic[24] and manganese, seem to exercise a stimulating action on bacterial activities; the causes of this action are not at present understood.
The acidity of the soil has an important effect on the bacterial processes. The acidity of soils may increase to such a point that the decomposition of plant tissues by bacteria is hindered, a peat layer being thus produced. The degree of acidity that is toxic varies very greatly with different soil bacteria, some of them, like Azotobacter and Nitrosomonas being very intolerant of acidity.
The conditions of aeration, water content, and temperature are inter-related in field soil. Ammonifying organisms are not greatly dependent on aeration, but this factor is sometimes a limiting one in the case of the very aerobic nitrifying bacteria. Hence efficient soil cultivation is beneficial to nitrification.
Many attempts have been made to correlate the temperature and moisture of field soils with the bacterial numbers and activities. These attempts have given very discordant results. It is generally agreed that a plentiful moisture supply is beneficial. Thus Greaves, in Utah, found the optimum water content for ammonia and nitrate production to be about 60 per cent. of the water-holding capacity. On the other hand, Prescott[56] found that the summer desiccation of soil in Egypt was followed by increased bacterial activities. Fabricius and Feilitzen,[18] using moor soil, found a direct relationship between soil temperature and bacterial numbers, showing that temperature can be a limiting factor under certain conditions. With normal arable soils, however, no such direct effect of temperature or moisture can be found[16] (see Fig. 8). It has even been found by Conn[11] that freezing of the soil may cause a marked increase in bacterial numbers. The erratic effects of temperature and moisture on the soil bacteria probably afford instances of a disturbance of the equilibrium between the bacteria and other[62] components of the soil micro-population. Thus desiccation and freezing, though they harmfully affect the bacteria, may inhibit other micro-organisms to a greater degree, thus freeing the bacteria from competition. It is in the investigation of[63] this equilibrium, and of the factors that can control it to our benefit, that the great advances in soil biology in the future are to be expected.
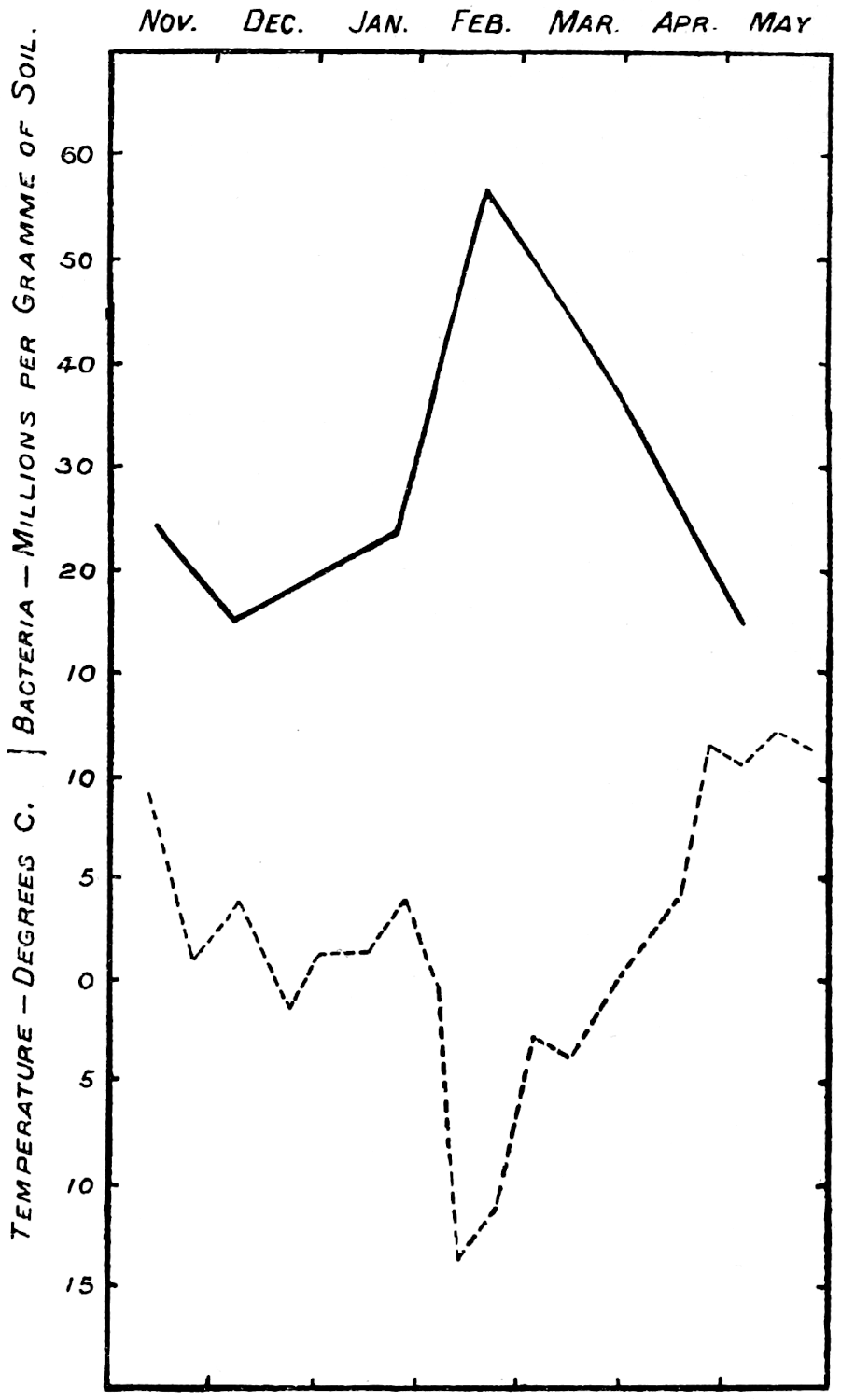
Fig. 8.—Effect of frost on the bacterial numbers in the soil. (After Conn.)
X-axis: Nov.-May
Y-axis (bottom): Temperature—Degrees C.
Y-axis (top): Bacteria—Millions per Gramme of Soil.
[1] Ashby, S. F., Journ. Agric. Sci., 1907, vol. ii., p. 35.
[2] Beijerinck, M. W., Centr. f. Bakt., 1900, Abt. II., Bd. 6, p. 1.
[3] Beijerinck, M. W., and Van Delden, A., Centr. f. Bakt., 1902, Abt. II., Bd. 9, p. 3.
[4] Bewley, W. F., and Hutchinson, H. B., Journ. Agric. Sci., 1920, vol. x., p. 144.
[5] Bonazzi, E., Journ. Bact., 1921, vol. vi., p. 331.
[6] Burrill, T. J., and Hansen, R., Illin. Exp. Sta., 1917, Bulletin 202.
[7] Christensen, H. R., Centralblatt. f. Bakt., 1915, Abt. II., Bd. 43, p. 1.
[8] Christensen, H. R., Centralblatt. f. Bakt., 1907, Abt. II., Bd. 17, pp. 109, 161.
[9] Christensen, H. R., and Larsen, O. H., Centralblatt. f. Bakt., 1911, Abt. II., Bd. 29, p. 347.
[10] Conn, H. J., Centralblatt. f. Bakt., 1910, Abt. II., Bd. 28, p. 422.
[11] Conn, H. J., Centralblatt. f. Bakt., 1914, Abt. II., Bd. 42, p. 510.
[12] Conn, H. J., Journ. Bact., 1916, vol. i., p. 187.
[13] Conn, H. J., Journ. Bact., 1917, vol. ii., p. 137.
[14] Conn, H. J., Journ. Bact., 1917, vol. ii., p. 35.
[15] Cramer, E., Arch. f. Hyg., 1893, Bd. 16, p. 151.
[16] Cutler, W., Crump, L. M., and Sandon, H., Phil. Trans. Roy. Soc., 1923, Series B, vol. ccxi., p. 317.
[17] Doryland, C. J. T., N. Dakota Agr. Exp. Sta., 1916, Bulletin 116.
[18] Fabricius, O., and Feilitzen, H., Centr. f. Bakt., 1905, Abt. II., Bd. 14, p. 161.
[19] Fisher, R. A., Thornton, H. G., and Mackenzie, W. A., Ann. Appl. Biol., 1922, vol. ix., p. 325.
[20] Fred, E. B., and Hart, E. B., Wisconsin Agr. Exp. Sta. Research, 1915, Bulletin 35.
[21] Gainey, P. L., Journ. Agric. Research, 1918, vol. xiv., p. 265.
[22] Golding, J., Journ. Agric. Sci., 1905, vol. i., p. 59.
[23] Greaves, J. E., Soil Sci., 1916, vol. ii., p. 443.
[24] Greaves, J. E., Journ. Agric. Res., 1916, vol. vi, p. 389.
[25] Greaves, J. E., Soil Sci., 1920, vol. x., p. 77.
[26] Greaves, J. E., and Lund, Y., Soil Sci., 1921, vol. xii., p. 163.
[27] Greaves, J. E., and Carter, E. G., Journ. Agric. Research, 1916, vol. vi., p. 889.
[28] Groenewege, J., Arch. Suikerindust., 1913, Bd. 21, p. 790.
[64]
[28b] Green, H. H., Union of S. Africa Dept. Agr., Rept. of Director Vet. Res., 1918, p. 592.
[29] Hutchinson, C. M., Rept. Agr. Res. Inst. and Col. of Pusa, 1912, p. 85.
[30] Hutchinson, H. B., and Clayton, J., Journ. Agric. Sci., 1919, vol. ix., p. 143.
[30b] Hutchinson, H. B., and Richards, H. H., Journ. Min. Agric., 1921, vol. xxviii., p. 398.
[31] Hanzawa, J., Centr. f. Bakt., 1914, Abt. II., Bd. 41, p. 573.
[32] Hopkins, C. G., and Whiting, A. L., Ill. Agr. Exp. Sta., 1916, Bulletin 190, p. 395.
[33] Hoppe-Seyler, G., Ztschr. Phys. Chem., 1886, vol. x, pp. 201, 401; 1887, vol. xi., p. 561.
[34] Hesselmann, H., Skogsvårdsför. Tidskr., 1917, No. 4, p. 321.
[35] Joshi, N. V., Mem. Dept. Agr. in India, Bact. Ser., 1920, vol. i., No. 9.
[36] Koch, R., Mitt. Kais. Gesundh., 1881, vol. i., p. 1.
[37] Kaserer, H., Centr. f. Bakt., 1906, Abt. II., Bd. 16, p. 681.
[38] Kaserer, H., Centr. f. Bakt., 1905, Abt. II., Bd. 15, p. 573.
[39] Krainskii, A. V., Centr. f. Bakt., 1910, Abt. II., Bd. 26, p. 231.
[40] Klimmer, M., and Kruger, R., Centr. f. Bakt., 1914, Abt. II., Bd. 40, p. 257.
[41] Krzeminiewski, S., Centr. f. Bakt., 1909, Abt. II., Bd. 23, p. 161.
[42] Koch, A., and Seydel, S., Centr. f. Bakt., 1912, Abt. II., Bd. 31, P. 570.
[43] Lipman, C. B., Bot. Gaz., 1909, vol. xlviii., p. 106.
[44] Lipman, C. B., and Burgess, P. S., Centr. f. Bakt., 1914, Abt. II., Bd. 41, p. 430.
[45] Lipman, C. B., and Burgess, P. S., Centr. f. Bakt., 1915, Abt. II., Bd. 44, p. 481.
[46] Lipman, C. B., and Waynick, D. O., Soil Sci., 1916, vol. i., p. 5.
[47] Löhnis, F., and Pillai, N. K., Centr. f. Bakt., 1908, Abt. II., Bd. 20, p. 781.
[47b] Löhnis, F., and Smith, T., Journ. Agric. Res., 1914, vol. vi., p. 675.
[48] Mackenna, J., Rept. Prog. Agric., India, 1917, p. 101.
[49] Marchal, E., Bull. Acad. Roy. Belgique, 1893, vol. xxv., p. 727.
[50] McBeth, I. G., and Scales, F. M., U.S. Dept. Ag., Bureau Plant Indus., 1913, Bulletin 266.
[51] Mockeridge, J., Biochem. Journ., 1915, vol. ix., p. 272.
[52] Nabokich, A. J., and Lebedeff, A. F., Centr. f. Bakt., 1906, Abt. II., Bd. 17, p. 350.
[53] Nagaoka, M., Bull. Coll. Agr., Tokyo, 1900, vol. vi., No. 3.
[54] Omelianski, W. L., Comptes Rendus Acad. Sci., 1895, vol. cxxi., p. 653; 1897, vol. cxxv., pp. 907, 1131; Arch. Sci. Bio., (St. Petersburg), 1899, vol. vii., p. 411.
[65]
[55] Omelianski, W. L., and Sohmskov, M., Arch. Sci. Biol., Publ. Inst. Imp. Med. Exp. (Petrograd), 1916, vol. xviii., pp. 327, 338, 459; vol. xix., p. 162.
[56] Prescott, J. A., Journ. Agr. Sci., 1920, vol. x., p. 177.
[57] Söhngen, N. L., Centr. f. Bakt., 1905, Abt. II., Bd. 15, p. 513.
[58] Sen Gupta, N., Journ. Agr. Sci., 1921, vol. xi., p. 136.
[59] Shearer, C., Journ. Hyg., 1919, vol. xviii., p. 337.
[60] Tappeiner, Ber. Deut. Chem. Gesell., 1883, vol. xvi., p. 1734; Zeitsch. Biol., 1884, vol. xx., p. 52.
[61] Thornton, H. G., Ann. Appl. Biol., 1922, vol. ix., p. 241.
[62] Wallin, I. E., Journ. Bact., 1922, vol. vii., p. 471.
[63] Waksman, S. A., and Joffe, J. S., Journ. Bact., 1922, vol. vii., p. 239.
[64] Wilson, J. K., Cornell Agric. Exp. Sta., 1917, Bulletin 386.
[66]
That protozoa could be isolated from the soil was a matter of common knowledge to the biologists of the nineteenth century, but not until the early part of the present century was it suggested that these organisms might be playing some part in the general economy of the soil micro-population. Of recent years a great deal of our knowledge of the cytology of the different groups of protozoa, especially the Amœbæ, has been obtained from the study of representatives normally living in the soil; but unfortunately little or no knowledge has been gained of the biology of these animals in their natural habitat.
The view that the presence of these organisms in excessive numbers may lead to “soil sickness” was first put forward by Russell and Hutchinson in 1909, and elaborated in their further papers dealing with “Partial Sterilisation of the Soil.”
It is unnecessary to discuss in detail this important branch of agriculture, but to obtain a clear idea of the development of the study of soil protozoa it is necessary to give as briefly as possible the conclusions deduced by Russell and Hutchinson from their extensive experiments on soils treated with steam and various volatile antiseptics[21], [22]:—
“(1) Partial sterilisation of the soil causes first a fall, then a rise, in bacterial numbers, which goes on till the numbers considerably exceed those present in the original soil.
“(2) Simultaneously there is a marked increase in the rate of accumulation of ammonia which is formed from organic nitrogen compounds.
[67]
“(3) The increase in bacterial numbers is the result of improvement in the soil as a medium for bacterial growth, and not an improvement in the bacterial flora.
“(4) The improvement in the soil brought about by partial sterilisation is permanent, the high bacterial numbers being kept up even for 200 days or more. It is evident from (3) and (4) that the factor limiting bacterial numbers in ordinary soil is not bacterial, nor is it any product of bacterial activity, nor does it arise spontaneously in soils.
“(5) But if some of the untreated soil is introduced into partially sterilised soil, the bacterial numbers, after the initial rise, begin to fall. Thus the limiting factor can be reintroduced from untreated soils.
“(6) Evidence of the limiting factor in untreated soils is obtained by studying the effect of temperature on bacterial numbers. Untreated soils were maintained at 10°, 20°, 30° C. in a well-moistened aerated condition, and periodical counts were made of the numbers of bacteria per gram. Rise in temperature rarely caused any increase in bacterial numbers. But after the soil was partially sterilised the bacterial numbers showed the normal increase with increasing temperatures.
TABLE VI.
| Temperature of Storage. °C. |
Untreated Soil. | Soil Treated with Toluene. | ||||||
|---|---|---|---|---|---|---|---|---|
| At Start. |
After 13 Days. |
After 25 Days. |
After 70 Days. |
At Start. |
After 13 Days. |
After 25 Days. |
After 70 Days. |
|
| 5°-12° | 65 | 63 | 41 | 32 | 8·5 | 73 | 101 | 137 |
| 20° | 65 | 41 | 22 | 23 | 8·5 | 187 | 128 | 182 |
| 30° | 65 | 27 | 50 | 16 | 8·5 | 197 | 145 | 51 |
| 40° | 65 | 14 | 9 | 33 | 8·5 | 148 | 52 | 100 |
“(7) It is evident, therefore, that the limiting factor in the untreated soils is not the lack of anything, but the presence of something active. The properties of the limiting factor are:—
“(a) It is active and not a lack of something.
“(b) It is not bacterial.
[68]
“(c) It is extinguished by heat or poisons.
“(d) It can be re-introduced into soils from which it has been extinguished by the addition of a little untreated soil.
“(e) It develops more slowly than bacteria.
“(f) It is favoured by conditions favourable to trophic life in the soil, and finally becomes so active that the bacteria become unduly depressed.
“It is difficult to see what agent other than a living organism can fulfil these conditions. Search was therefore made for a larger organism capable of destroying bacteria, and considerable numbers of protozoa were found. The ciliates and amœbæ are killed by partial sterilisation. Whenever they are killed the detrimental factor is found to be put out of action; the bacterial numbers rise and maintain a high level. Whenever the detrimental factor is not put out of action, the protozoa are not killed. To these rules we have found no exception.”
From such premises as the above Russell and Hutchinson founded the “protozoa theory of partial sterilisation,” and at Rothamsted there was commenced the serious study of these soil organisms.
Goodey was one of the early workers on this new subject, and added considerably to our knowledge of the species living in normal soils, and of the chemical constitution of the cyst wall of ciliates. He also made investigations on the effects of various chemicals on the micro-population of soils, but was unable to draw very definite conclusions.[11]
One of the first criticisms raised against the protozoa theory of partial sterilisation was that the protozoa were not normal inhabitants of the soil, and were present only in small numbers, all of them in the cystic, quiescent condition. It was further held that these cysts were carried by the wind from dried-up ponds and streams. It is difficult to trace the origin of this view, since early observers, viz., Ehrenberg and Dujardin, in 1841, were of the opinion that the protozoa[69] were living in the trophic active condition in the soil, and it was not until 1878 that Stein showed that free living protozoa can encyst. To Martin and Lewin, however, must be ascribed the distinction of first proving that the soil possesses an active protozoan population, for by a series of ingenious experiments these observers isolated several flagellates and amœbæ in a trophic condition from certain of the Rothamsted soils.[18] The more recent work in this country has been in the direction of devising new quantitative methods of research, since by this means alone is it possible to elucidate many fundamental questions.
In America and elsewhere experiments have been devised for testing the conclusions of Russell and Hutchinson. Cunningham and Löhnis,[2] in America, Truffaut and Bezssonoff,[24] in France, supply evidence in favour of the theory, but most of the American work is in opposition to it.
Sherman[23] is perhaps the most prominent in opposing the phagocytic action of protozoa on soil bacteria in spite of the fact that certain of his experimental results apparently show enormous decreases in bacterial numbers in the presence of protozoa. In many of his soil inoculation experiments, however, it was not demonstrated that his active cultures remained alive after entering the soil.
The experimental difficulties of dealing with soil protozoa are considerable, and without a thoroughly sound technique investigators may easily go astray.
The animal kingdom is divided into two main groups or sub-kingdoms—the Protozoa and the Metozoa. In the latter the characteristic feature is that the body is composed of several units, called cells, and consequently such animals are often spoken of as multicellular. The Protozoa, on the other hand, are usually designated as uni-cellular, since their bodies are regarded as being homologous to a single unit or cell of the metozoan body. For various reasons exception has been taken by Dobell[9] and others to the use of the term uni-cellular,[70] for, as Dobell says, “If we regard the whole organism as an individual unit, then the whole protozoan is strictly comparable with a whole metozoon, and not with a part of it. But the body of a protozoan, though it shows great complexity of structure, is not differentiated internally into cells, like the body of a metozoon. Consequently it differs from the latter not in the number of its cellular constituents, but in lacking these altogether. We therefore define the sub-kingdom of the protozoa as the group which contains all non-cellular animals.”
It should be pointed out that this view does not find favour with many zoologists, but it is useful in bringing into prominence the fact that each protozoan is comparable as regards its functions with the multi-cellular animals.
The protozoa are again further divided into four main classes:—
| I. | Rhizopoda. |
| II. | Mastigophora. |
| III. | Ciliophora. |
| IV. | Sporozoa. |
Of the above classes, representatives of each of the first three are found living in the soil, but up to the present there is no evidence that any sporozoon is capable of living an active life in the soil, though the cysts of such organisms may be present.
The class RHIZOPODA consists of those protozoa whose organs of locomotion and food capture are pseudopodia, that is, temporary extensions of the living protoplasm. The body is typically naked, that is to say, without any cuticular membrane, though in some forms, ex. Amœbæ terricola, the external layer of protoplasm is thickened to form a pellicle. A skeleton or shell may be present.
The class is further sub-divided into various sub-classes, only two of which concern the soil protozoologist, viz., the Amœbæ and the Mycetozoa, of which the most important representative is Plasmodiophora brassicæ, which attacks the[71] roots of many cruciferous plants, causing the disease familiarly known as “Fingers and Toes.”
The Amœbæ are again divided into two orders:—
(a) Nuda, without shell or skeleton;
(b) Testacea, with shells often termed Thecamœbæ.
Representatives of the “naked” amœbæ commonly found in soils are Nægleria (Dimastigamœba) gruberi, Amœba diploidea (possessing two nuclei) and A. terricola, the last two forms possessing a comparatively thick skin or pellicles. Trinema enchelys, Difflugia constricta and Chlamydophrys stercorea are examples of soil Thecamœbæ.
The class MASTIGOPHORA consists of those protozoa whose typical modes of progression are by means of flagella, whip-like filaments which, by their continual lashing motion, cause movement of the animal.
The body may be naked or corticate. The only organisms which concern the soil biologist belong to the Flagellata order.
The Flagellates differ considerably among themselves, both as regards their mode of feeding, and the number of flagella, thus making their classification difficult and outside the scope of this book. Suffice it to say that in the soil such organisms occur possessing one, two, three or four flagella, ex. Oicomonas termo, Heteromita globosus, Dallengeria and Tetramitus spiralis. Further, their mode of feeding may be saprophytic in which nourishment is absorbed by diffusion through the body surface in the form of soluble organic substances, holozoic where solid food particles are taken in, or holophytic in which food is synthesised by the energy of sunlight. This last group is commonly spoken of as the Phyto flagellates, which are to all intents and purposes unicellular algæ, and as such will be dealt with in Chapter VI.
The class CILIOPHORA consists of those protozoa whose typical organs of locomotion are threads or cilia. These organisms can in one sense be regarded as the highest of the protozoa, since in no other division does the body attain so great a complexity of structure. Moreover, they are[72] typically characterised by a complicated nuclear apparatus with the vegetative and generative portions separated into distinct bodies, the macro-nucleus and the micro-nucleus. Their mode of nutrition is holozoic, though recently Peters has brought forward evidence that certain species can obtain their nourishment saprophytically.
The sub-class Ciliata comprises four orders, all of which are represented in the soil.
I. Holotricha. The cilia are equal in length and uniformly distributed over the whole body in the primitive forms, though restricted to special regions in the specialised forms. Typical soil forms are Colpoda cucullus, Colpidium colpoda.
II. Heterotricha. There is a uniform covering of cilia, and a conspicuous spiral zone of larger cilia forming a vibratile membrane and leading to the mouth.
III. Hypotricha. The body is flattened dorso-ventrally and the cilia are often fused to form larger appendages or cirri confined to the ventral surface. Movement is typically a creeping one. Typical soil forms are Pleurotricha, Gastrostylis, Oxytricha.
IV. Peritricha. Typically of a sedentary habit and the cilia are reduced to a zone round the adoral region of the body. A typical soil form is Vorticella microstomum.
The above classification is far from complete, but should be sufficient to give an idea of the general grouping of the organisms. For a more detailed account reference must be made to the numerous text books on protozoa.
The life history of each species has its own characteristic features as regards nuclear division, etc., and in many forms, notably the amœbæ, it is impossible to identify them with certainty unless the chief stages of the life history are known. In general, however, the soil protozoa pass through very similar phases and develop in a perfectly straightforward way. Broadly speaking, there are two main phases of the[73] life history—a period of activity often mistermed vegetative, and a period of rest. In the former the animal moves, feeds and reproduces, while in the latter there is secreted round the body a thick wall, capable of resisting adverse external influences. This condition is termed the cystic stage, and by means of it the animals are distributed from place to place by air, water, etc. Indeed, so resistant are the cysts that many of them are capable of withstanding the action of the digestive juices of the intestines of animals, through which they pass to be deposited by the fæces on fresh ground.
This cystic stage of the life history is found in practically all free-living protozoa, though it is not formed in exactly the same manner in every case. In the majority of instances the cyst is the product of a single organism, round which is formed a delicate gelatinous substance which soon hardens and gradually acquires the peculiar characters of the wall. Concerning the chemical nature of this wall there is little known, but Goodey,[11] working on the cysts of Colpoda cucullus, found it to be formed of a carbohydrate, different from all carbohydrates previously described, to which the name “Cytose” was given. When in this state the animals are able to remain dormant for considerable periods until favourable conditions once more obtain when the wall is ruptured and the animal again resumes the active phase of its life history. This simple process is characteristic of such species as Heteromita globosus, Cercomonas spp., and many others. It will be noted that no increase of numbers, i.e. reproduction, occurs. A more complex condition is, however, sometimes found, as, for example, in the ciliate Colpoda steinii, where actual reproduction into small animals takes place within the cyst.
Finally there is the less common type of cyst formation, such as is found in the flagellate Oicomonas termo described by Martin.[19] This flagellate, in common with all other forms, reproduces by dividing into two; the division of the nucleus initiating the process. At certain undetermined periods of[74] the life history, however, conjugation occurs between two similar animals forming a large biflagellate body known as the zygote. After swimming about for varying periods of time, during which the size increases and a large vacuole appears, the zygote secretes a thick wall, loses its flagella, and becomes a cyst. While in this condition the two gamete nuclei fuse to form one, and eventually a single Oicomonas emerges from its cyst.
Similarly in A. diploidea the cysts are formed after two individuals have come together. In the young cysts two amœbæ are found in close association, and according to Hartmann and Nägler[12] a sexual process occurs inside the cyst involving a “reductive” division of the nuclei. This requires confirmation, but it is certain that only one individual comes out of the cysts, which originally contained two amœbæ.
Such cysts have been termed by some writers “reproductive,” evidently a misleading term, since no increase in numbers, but rather a decrease, results from the process. A better term is, perhaps, conjugation cyst.
In soil protozoa, then, three different modes of cyst formation obtain, and failure to make the distinction inevitably leads to confusion.
Before leaving the question of life histories, reference must be made to a peculiar and characteristic feature of Nægleria gruberi. This amœba under certain circumstances assumes a free-swimming biflagellate stage. After variable periods of time the flagella are lost and the ordinary amœboid condition resumed. What are the factors concerned in the production of flagellates is unknown, but flooding the coverslips with distilled water is an effective method for causing their appearance.
For both the bacteria and algæ observations have been made regarding their distribution through successive depths[75] of the soil; little can be said, however, about the protozoa in this connection. It is certain that they occur throughout the first six inches of the Rothamsted soils, though their relative frequencies in the successive inches has not been determined, but probably they are most abundant in the 2nd to the 4th inch.
In this country experiments have not been made to determine whether sub-soil normally contains protozoa; but from some South African soil, taken under sterile conditions 4 ft. down and examined in this laboratory, large numbers of protozoa were cultivated.
This soil, however, could not, for various reasons, be regarded as a typical sub-soil.
Kofoid records the presence of Nægleria gruberi in clay and rock talus taken from the sides of excavations of over 20 ft. depth, but the possibility of external infection does not appear to have been excluded.
The presence of protozoa is not peculiar to British soil since they have been found by various workers in Germany, France, the United States, and elsewhere. In view of their probable importance in the soil economy there has been instituted a survey of the protozoan species of soil from all parts of the world.
This work is in charge of Mr. Sandon, to whom I am indebted for the following summary of his as yet unpublished research.
“The majority of soil protozoa (like the fresh-water forms) appear to be quite cosmopolitan, for the species found in such widely separated localities as England, Spitsbergen, Africa, West Indies, Gough Island (in the South Atlantic) and Nauru (in the Pacific) are, with few exceptions, identical. This distribution indicates an ability to withstand an extremely wide range of conditions, for the same species occurring in Arctic soils, which are frozen for the greater part of the year, are found also in soils exposed to the direct rays of the tropical sun. Even sand from the Egyptian desert contains protozoa, though it seems probable[76] that in such cases they must be present only in the encysted condition for the greater part of the time.
“Not every sample of soil, however, contains all the species capable of living in soil, but the local conditions determining the presence or absence of any species are at present unknown. In general the numbers, both of species and of individuals present, follow the number of bacteria. They are consequently most numerous in rich moist soils. The statement sometimes made that protozoa are most numerous in peaty soils is based solely on the number of Rhizopod shells found in such localities; but as most of these shells are empty, their abundance is probably due simply to the slowness with which they disintegrate in these soils where bacterial activity is low, they do not indicate a great protozoal activity. Active protozoa do occur even in extremely acid soils, but their numbers in such cases are low. The common soil protozoa, in fact, appear to be as tolerant of differences in soil acidity as they are of differences in climate, for many of the same forms which occur in acid soils are found also in soils containing high percentages of chalk. It is possible that some of the less common species may be confined within closer limits of external conditions but the information available on this point is inadequate. All the species, however, which in Rothamsted soils occur in the highest numbers (e.g. Oicomonas termo, Heteromita spp., Cercomonas crassicauda, Nægleria gruberi, Colpoda cucullus, C. steinii) occur in practically every soil which is capable of supporting vegetation, though, of course, in very varying numbers.”
It is evident, therefore, that the protozoa must be regarded as constituting part of the normal micro-organic population of soils, and as such are probably playing an important rôle. Unfortunately our knowledge of the physiology of these organisms is extremely scant, and much of future research must be directed towards elucidating their functions and their responses to varying environmental conditions.
[77]
In the preceding chapter an outline has been given of the development of the study of soil protozoa, with especial reference to its qualitative aspects.
Here it is proposed to deal with the quantitative methods which have been devised for studying these organisms and the results obtained.
From the beginning great difficulty has been encountered in finding means for counting protozoa; and most of the early results have been obtained by the use of one of the following methods: (1) direct counts in a known volume of soil suspension by means of a microscope; (2) dilution method as used for counting bacteria, and suggested by Rahn, who made dilutions of the soil and determined, by examination at periodic intervals, the one above which protozoa did not grow; (3) Agar plating as used by Killer; (4) counting per standard loop of suspension as devised by Müller. Of these the two last have been little used, and for various reasons are now discarded by most workers. Direct methods have been used extensively in the United States by Koch[13] and others,[16] who claim to have got satisfactory results; they are, however, highly inaccurate and should be discontinued. The present writer[3] has shown that there exists a surface energy relationship between the soil particles and the protozoa, so that the two are always in intimate contact; thus rendering it impossible to count under the microscope the number of organisms in a given weight of soil suspension (Fig. 9). Further, in a clay soil, such as is found at Rothamsted, the clay particles alone make it very difficult to use such methods.
[78]
The demonstration of this surface energy relationship affords an effective rejoinder to the criticism made against Russell and Hutchinson’s hypothesis, viz., that soil protozoa must be very few in numbers, since it was impossible to see them on examining soil under the microscope.
Fig. 9.—Showing the number of amœbæ and flagellates withdrawn from suspensions of varying strengths by different types of solid matter. A = clay: B = partially sterilized soil: C = ignited soil: D = fine sand: E = waste sand. Since complete withdrawal occurs when the numbers of organisms added are less than the capacity of the solid matter, the first part of each of the above curves is coincident with the ordinate. The numbers of organisms are given in thousands. (From Journ. Agric. Soc., vol. ix.)
X-axis: Number of Organisms per c.c. left in Solution.
Y-axis: Number of Organisms per c.c. taken up by Solid Matter.
The second or dilution method is the one, therefore, that has been most extensively developed.
[79]
Cunningham obtained concordant results in this way, and his method, modified by L. M. Crump, was as follows: 10 grams of soil were added to 125 c.c. of sterile tap-water and shaken for three minutes. This gives a 1 in 12·5 dilution. From it further dilutions were made until a sufficiently high one was obtained. Petri dishes, containing nutrient agar, were inoculated with 1 c.c. of each of the dilutions and incubated. At intervals covering 28 days the plates were examined and the presence or absence of protozoa on each recorded. In this way the approximate number of organisms per gram of soil could be found.
By methods essentially similar to this numerous counts have been made of the bacteria and protozoa in field soil and in partially sterilized soils. They were, however, inconclusive; thus, on the one hand, Goodey and several American observers, found no correlation between the numbers of protozoa and bacteria, while Miss Crump and Cunningham obtained evidence pointing to the reverse conclusion.
Such divergence of opinion was probably mainly due to two causes: firstly, that the time elapsing between the successive counts was too long, for it has been shown recently that the number of bacteria and protozoa fluctuate very rapidly; and secondly, the method was not completely satisfactory since only the total numbers of protozoa were considered, no means having been found of differentiating between the cystic and active forms. This was a particularly serious source of error for it is possible for soil to contain large numbers of bacteria and protozoa, of which a high percentage of the latter are in the form of cysts. A count made on such a soil would give results apparently opposed to the theory that protozoa act as depressors of bacteria.
This difficulty has, however, been overcome by a further modification of the dilution method, and it is now possible in any soil sample to count both the numbers of cysts and active forms. Also a further advance in technique has made it possible to recognise and enumerate the common species[80] of protozoa, instead of simply grouping them as Ciliates, Flagellates, and Amœbæ, as was done in the past.[7]
Briefly the method consists in dividing the soil sample into equal portions (usually 10 grams each) one of which is counted, thus giving the total numbers of protozoa (active + cystic) present. The second portion is treated over-night with 2 per cent. hydrochloric acid, the HCl used being B.P. pure 31·8 per cent. Previous experiments have shown that such acid kills all the active protozoa, leaving viable the cysts. The number of cysts is therefore found by counting this treated sample, and the number obtained subtracted from the total gives the active number.[F]
[F] The proof of the accuracy of this method will be found in the following papers:—
(1) Cutler, D. W. (1920), Journ. Agric. Sci., vol. x., 136-143.
(2) Cutler, D. W., and Crump, L. M. (1920), Ann. App. Biol., vol. vii., 11-24.
The discovery of this method at once puts into the hands of the investigator a much more efficient instrument for studying the activities of the soil micro-population, especially since at a slightly later date Thornton’s method for counting bacteria was devised.
Early in 1920 Cutler and Crump[6] decided to make a preliminary survey of the protozoon and bacterial populations of one of the Rothamsted field soils (Broadbalk dunged plot). The investigation was continued for 28 days, daily soil samples being taken. The results so obtained showed that an extended investigation of the micro-population of field soil would yield interesting and important results, especially as it was evident that certain views held by soil biologists required modification.
In July of the same year, therefore, it was decided to start an extended investigation of the soil protozoa and bacteria. The method adopted was to make counts of the numbers of bacteria and of six[G] species of protozoa in soil samples taken daily direct from the field (Barnfield dunged plot) and by statistical methods to correlate these counts one with another and with the data for external conditions. Observations at shorter periods than 24 hours could not be made, but it was found possible to continue the research for 365 days.[7]
[G] Actual counts were made of six species, though, as stated on p. 10, observations were made on seventeen.
[81]

Fig. 10.—Daily numbers of active amœbæ (Dimastigamœba and Species α) and bacteria in 1 gram of field soil, from August 29 to October 8, 1920. (From Phil. Trans. Roy. Soc., vol. ccxi.)
X-axis: August September October
Y-axis (left): Amoebae Active numbers per gramme of soil
Y-axis (right): Bacteria in millions per gramme of soil
Legend: Dimastigamoeba
Species α
Bacteria
[82]
The number of all the organisms showed large fluctuations of two kinds, daily and seasonal. The size of the changes that took place within so short a period as 24 hours was, perhaps, the most surprising fact that the experiment revealed. Thus three consecutive samples gave 58·0, 14·25 and 26·25 millions of bacteria per gram respectively; and the changes exhibited by any of the species of protozoa were at times even larger. This fact is of extreme importance, since in the past it has always been assumed that the number of bacteria remained fairly constant from day to day, and investigators have not hesitated to separate the taking of soil samples by long periods. It is now obvious that such a procedure is of little use for comparative purposes (Fig. 10).
It has usually been assumed that the changes in the external conditions markedly affect the density of the soil population. To test this the environmental conditions—temperature, moisture content and rainfall were examined; but contrary to all expectation no connection could be traced between any of these and the daily changes in numbers of any of the organisms investigated, and moreover the species of protozoa appeared in the main to be living independently of one another.
It is difficult to believe that external conditions are as inoperative as appears from the above; and in view of the known complexity of the soil it is possible that further research will show that certain combinations of external conditions are important agents in effecting the changes.
[83]
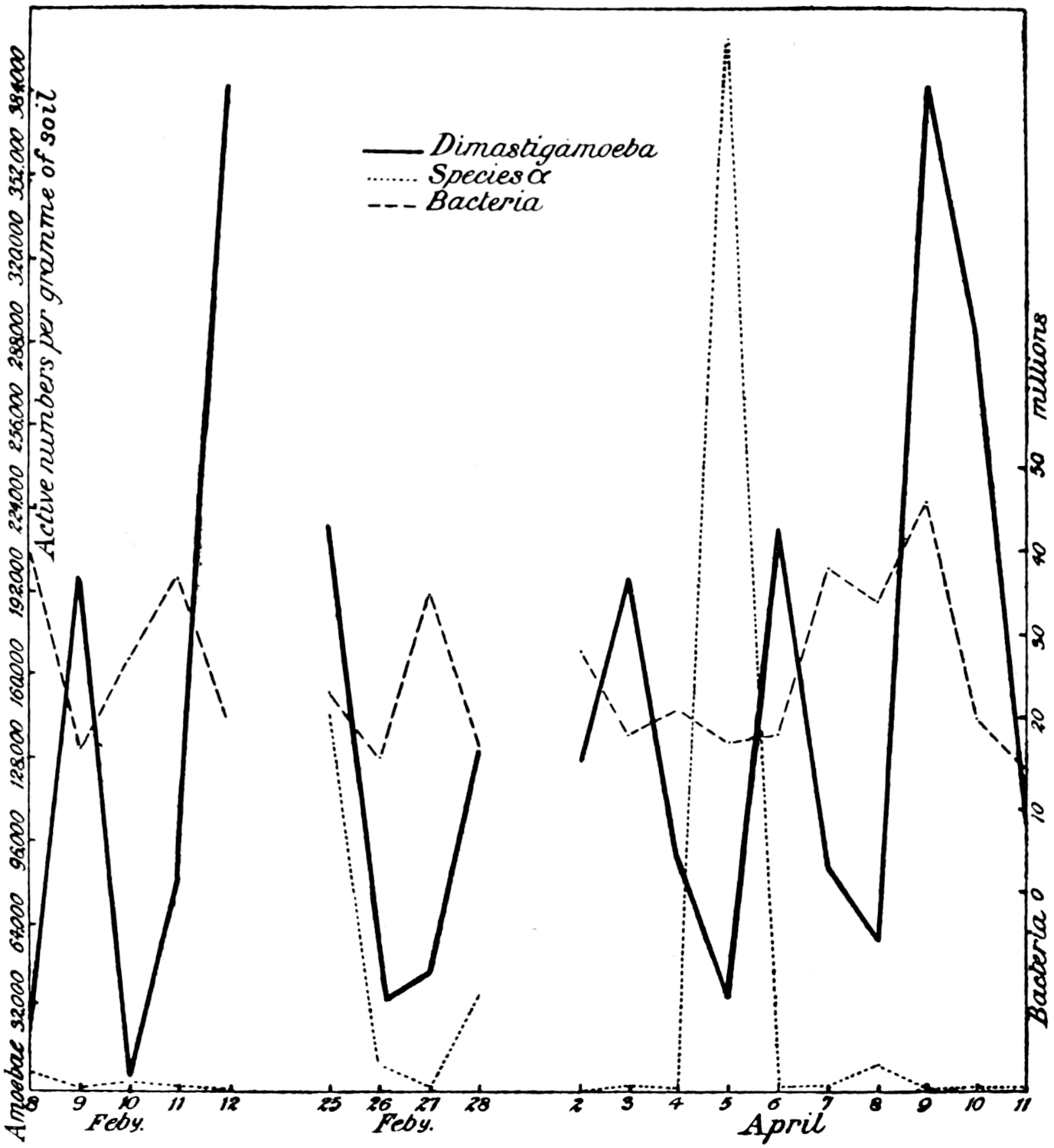
Fig. 11.—Numbers of active amœbæ (Dimastigamœba and Species α) and bacteria to 1 gram of field soil for typical periods in February and April, 1921. (From Phil. Trans. Roy. Soc., vol. ccxi.)
X-axis: Feby. Feby. April
Y-axis (left): Amoebae Active numbers per gramme of soil
Y-axis (right): Bacteria millions
Legend: Dimastigamoeba
Species α
Bacteria
In the case of the bacteria, however, the agent causing the fluctuations is mainly the active amœbæ. This was well shown during the year’s count, for with only 14 per cent. of exceptions, 10 per cent. of which can be explained as due to rapid excystation or encystation, a definite inverse relationship was established between the active numbers of amœbæ and the number of bacteria (Figs. 11 and 12). Thus a rise from one day to the next in the amœbic population was correlated with a fall in the numbers of bacteria and vice versa. It must not be supposed that the flagellates are of no account in this process; some species, known to eat bacteria, undoubtedly induce slight depressions, but,[84] owing to their small size, any effect is masked by the greater one of the amœbæ.
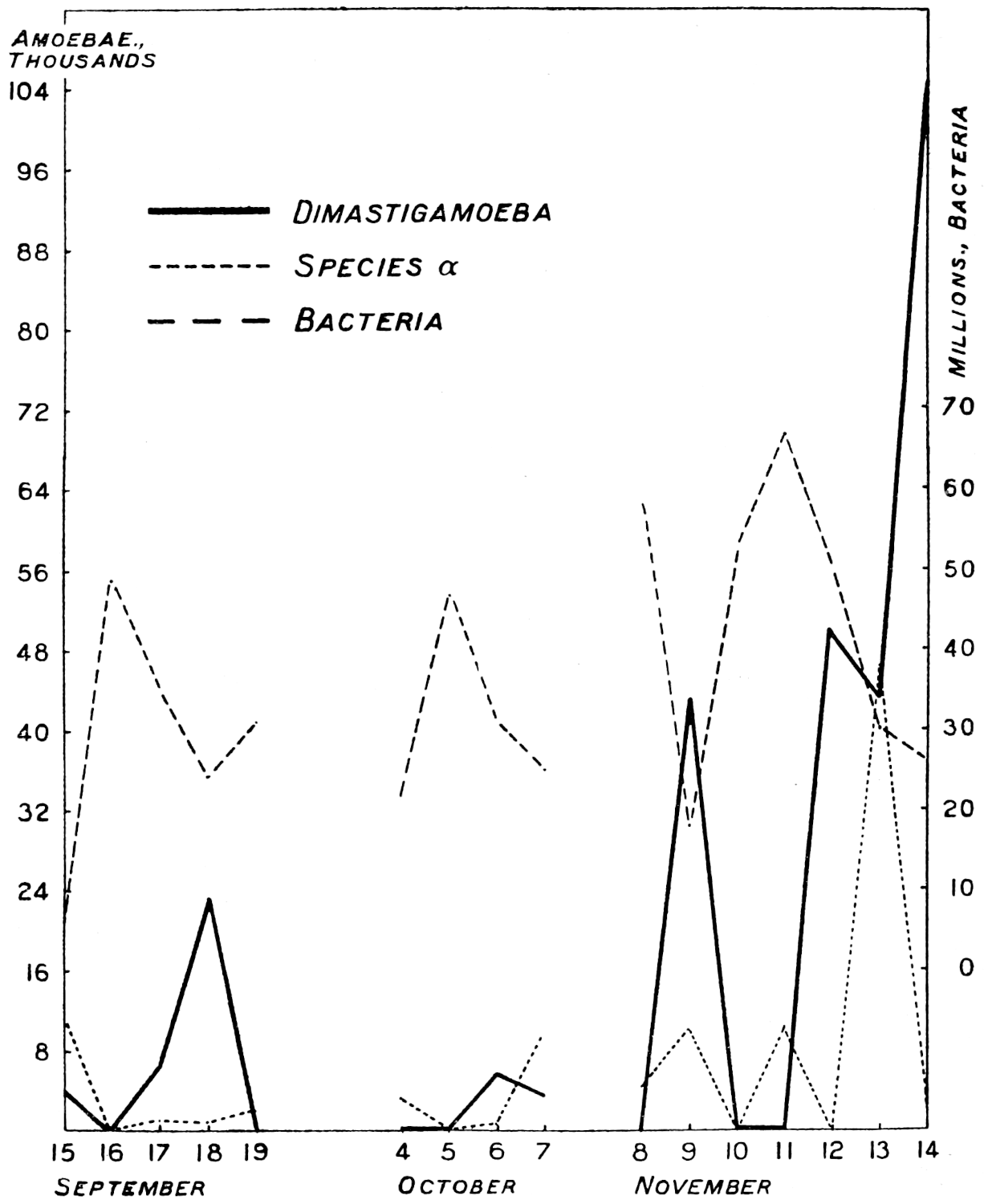
Fig. 12.—Numbers of active amœbæ (Dimastigamœba and Species α) and bacteria in 1 gram of field soil for typical periods in September, October, and November, 1920.
X-axis: August September October
Y-axis (left): Amoebae, thousands
Y-axis (right): Millions, Bacteria
Legend: Dimastigamoeba
Species α
Bacteria
These experiments seem to admit of no doubt that in field soil the active protozoa are instrumental in keeping down, below the level they might otherwise have attained,[85] the numbers of bacteria; but a further proof of this contention ought to be obtained by inoculation experiments. It should be possible, by inoculating sterile soil with bacteria alone and with bacteria plus protozoa, to demonstrate fluctuations in bacterial numbers in the latter, while those of the former remained constant. This admittedly crucial test has often been tried, but owing to difficulties in technique, etc., has always failed. Recently, however, by using new methods confirmatory results have been obtained.[5]
Ordinary field soil was sterilised by heat at 100° C. for 1 hour on four successive days; it was then divided into equal portions, one of which was inoculated with three known species of bacteria, and the other inoculated with the same number of bacteria plus the cysts of the common soil amœba Nægleria gruberi. The numbers of bacteria in each soil were counted daily for the first eight days and then daily from the 15th to the 21st day after the experiment started. The results are given in Table VII. and Fig. 13.
TABLE VII.
| Numbers of Days after Inoculation. |
Control (Bacteria alone). |
Control Bacteria + Amœbæ. |
|---|---|---|
| 0 | 13·0 | 12·2 |
| 1 | 48·6 | 35·4 |
| 2 | 97·6 | 117·2 |
| 3 | 127·0 | 178·4 |
| 4 | 154·8 | 154·4 |
| 5 | 196·8 | 177·0 |
| 6 | 214·4 | 151·8 |
| 7 | 193·4 | 75·6 |
| 8 | 165·2 | 65·8 |
| 15 | 169·2 | 72·8 |
| 16 | 174·8 | 30·2 |
| 17 | 175·6 | 53·2 |
| 18 | 168·4 | 82·8 |
| 19 | 160·4 | 43·8 |
| 20 | 171·2 | 70·8 |
| 21 | 176·2 | 28·2 |
| The numbers of bacteria are given in millions per gram of soil. |
||
[86]
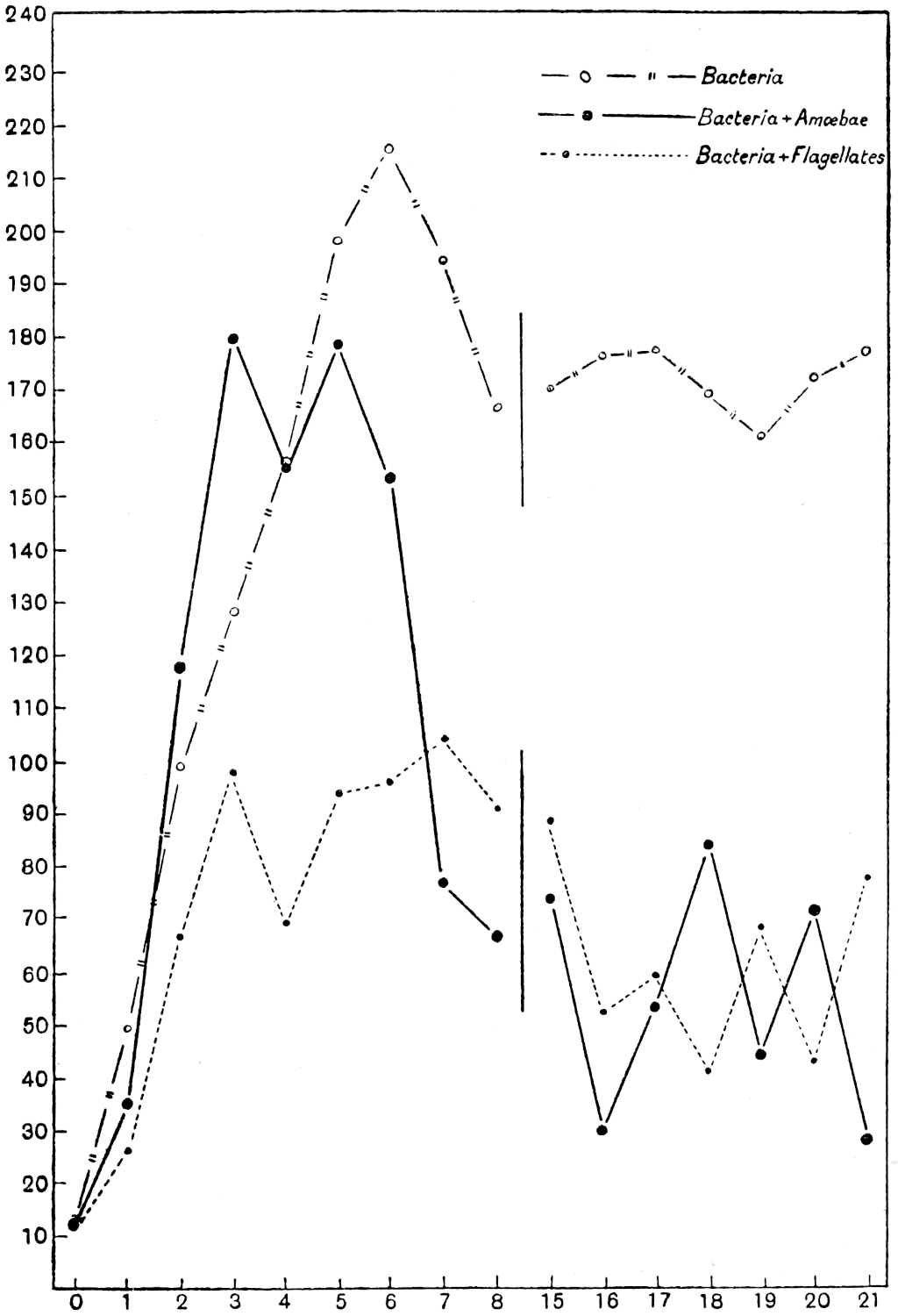
Fig. 13.—Numbers of bacteria counted daily in soils containing
A. Bacteria alone.
B. Same Bacteria as in A + Amœbæ.
C. Same Bacteria as in A + Flagellates.
(From Ann. Appl. Biol., vol. x.)
[87]
It will be noted that the numbers of bacteria in each soil rose steadily until a maximum was reached 6-8 days after inoculation. This is in accordance with expectation, since the reproductive rate of bacteria is much greater than that of the amœbæ, which, until their active forms are numerous, will not exert any appreciable influence on the bacterial population. Further, since the protozoa were inoculated as cysts an appreciable time would elapse before excystation took place. The last seven days of the experiment are of particular interest. During this period the amœbæ were known to be active in the soil, and were depressing the bacterial numbers, for in the control (protozoa-free) soil the variation in numbers was within experimental error, while in the other soil the variations were considerable and well outside experimental error. In fact the variations were comparable with those found from day to day in untreated field soils. Finally, the experiment shows that the bacteria in protozoa-free soil are able to maintain high numbers for a longer period than those living in association with protozoa.
Superimposed on the daily variations in numbers there are seasonal changes, as is clearly shown when fourteen day averages are made of the numbers for each species. Bacteria have long been known to show autumn and spring rises, but recent research has demonstrated that the protozoan population also rises to a maximum at the end of November, with a less marked spring rise at the end of March and beginning of April (Figs. 14 and 15).
It has sometimes been claimed that the numbers of soil organisms are closely linked with the soil moisture, but no support for this view was found during the course of the experiment. Similarly, as in the case of the daily variations, no connection could be traced between the seasonal changes and any of the external conditions considered.
It is interesting to note, however, that the seasonal variations in the numbers of soil organisms is very similar to those recorded for many aquatic organisms. Miss Delf,[8] for[88] instance, found that in ponds at Hampstead the algæ are most numerous in spring and again in the autumn, and like changes are recorded in British lakes by West and West[25] and in the Illinois river by Kofoid.[14]
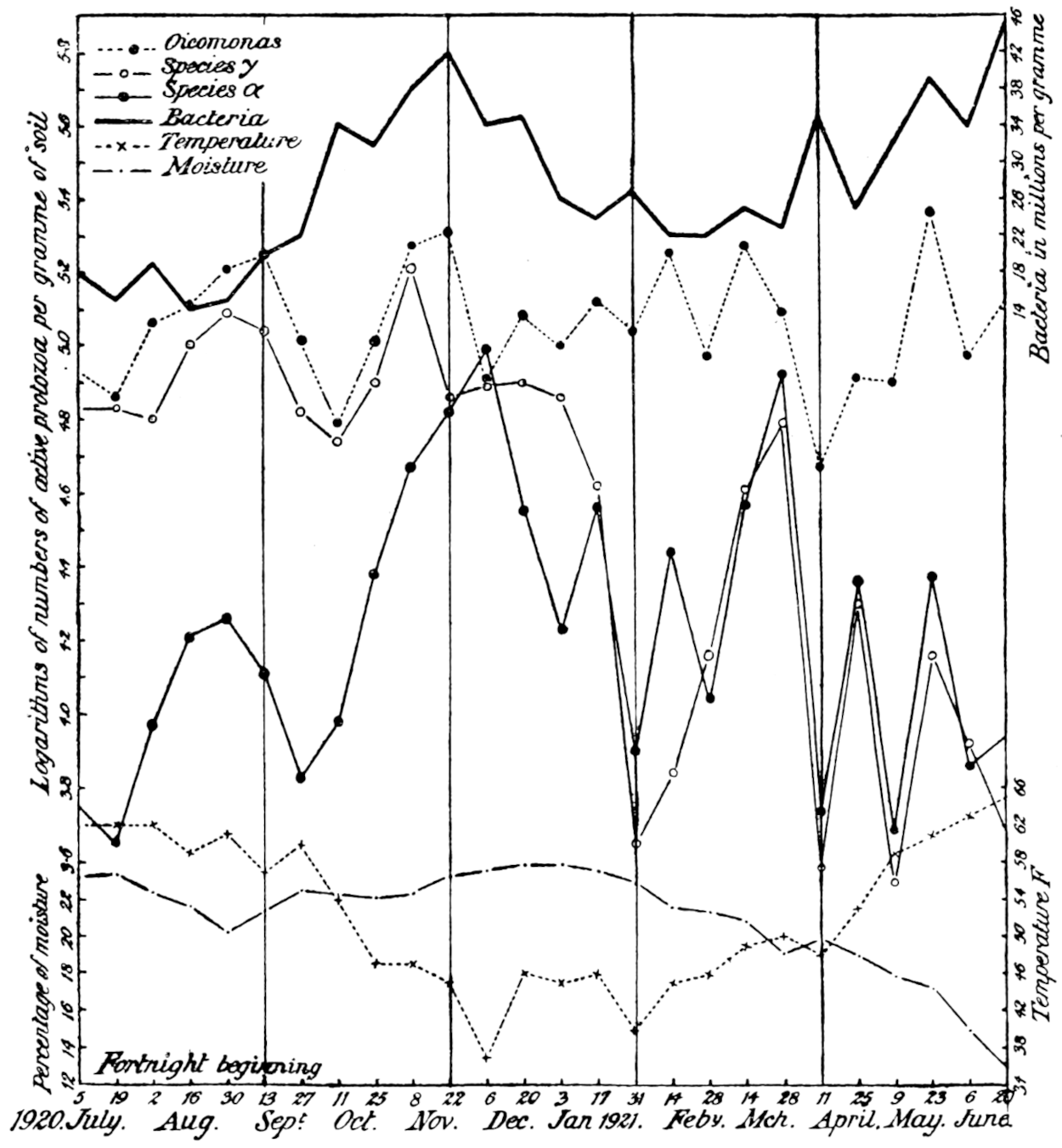
Fig. 14.—Fortnightly averages of total numbers of Oicomonas, Species γ, and Species α, and of bacteria, moisture, and temperature. (From Phil. Trans. Roy. Soc., vol. ccxi.)
X-axis: Fortnight beginning 1920. July. Aug. Sept. Oct. Nov. Dec. Jan 1921. Feby. Mch. April. May. June.
Y-axis (bottom left): Percentage of moisture
Y-axis (top left): Logarithms of numbers of active protozoa per gramme of soil
Y-axis (bottom right): Temperature F
Y-axis (top right): Bacteria in millions per gramme
Legend: Oicomonas
Species γ
Species α
Bacteria
Temperature
Moisture
It is difficult to resist the conclusion that these annual variations are produced by similar causes, from which it follows that the increase in the numbers of protozoa in the soil is not wholly conditioned by an increased food supply—the[89] bacteria—for the algæ are not dependent on such a form of nourishment. This is substantiated by the fact that the numbers of protozoa, except those of Oicomonas, rose during March, whereas the corresponding increase in the bacteria was delayed till the early part of April.
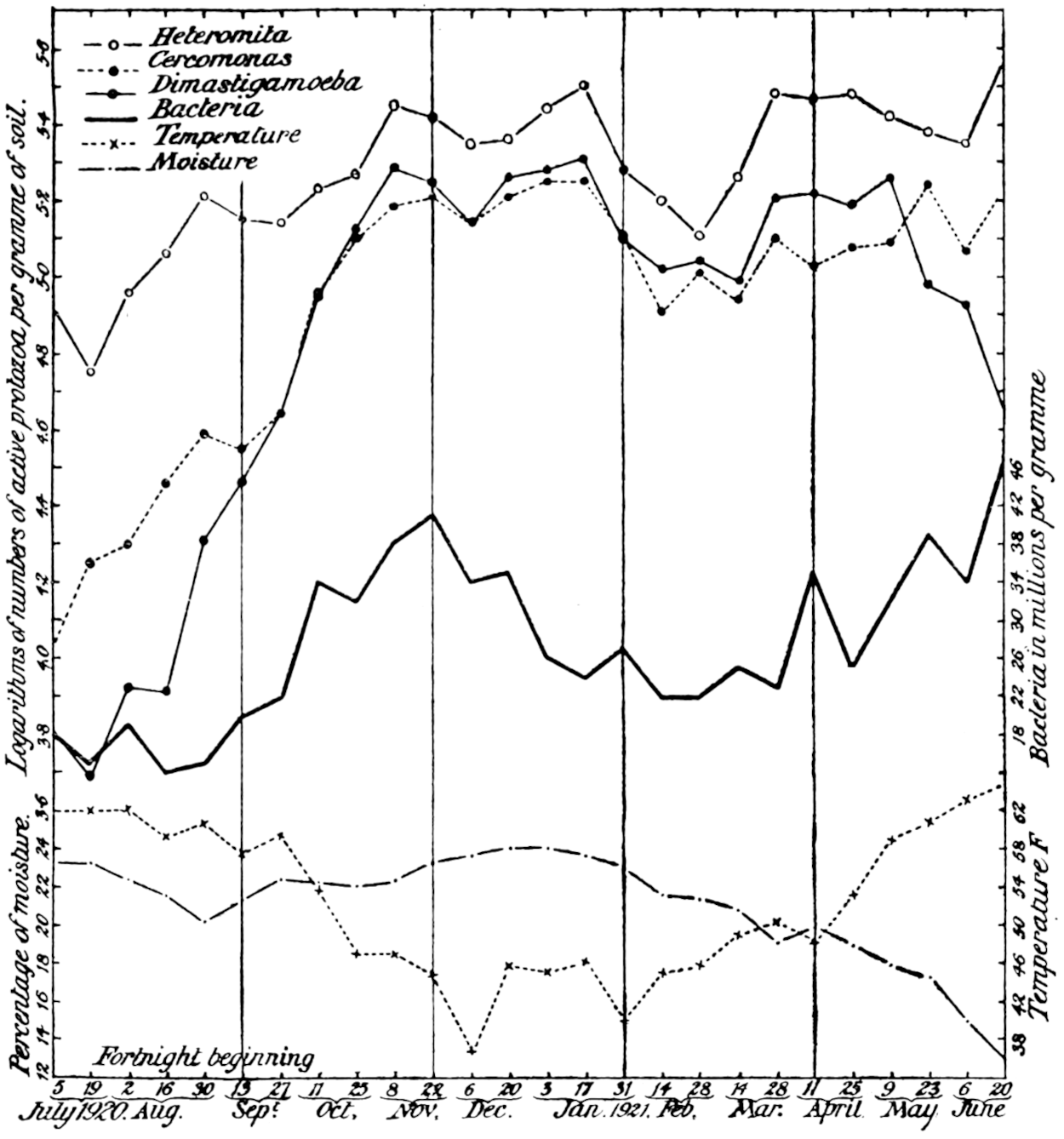
Fig. 15.—Fortnightly averages of total numbers of Heteromita, Cercomonas, and Dimastigamœba and of bacteria, moisture, and temperature. (From Phil. Trans. Roy. Soc., vol. ccxi.)
X-axis: Fortnight beginning July 1920. Aug. Sept. Oct. Nov. Dec. Jan. 1921. Feb. Mar. April May June
Y-axis (bottom left): Percentage of moisture.
Y-axis (top left): Logarithms of numbers of active protozoa per gramme of soil.
Y-axis (bottom right): Temperature F
Y-axis (top right): Bacteria in millions per gramme
Legend: Heteromita
Cercomonas
Dimastigamoeba
Bacteria
Temperature
Moisture
Owing to the variations in the numbers of both protozoa and bacteria, little reliance can be placed on figures obtained from an isolated count, since on one day the total numbers of[90] flagellates may be nearly 2,000,000 per gram and drop by more than half this figure in 24 days. It is certain, however, that the numbers recorded in the past are much too low, since the total flagellate and amœbæ species were lumped together in two groups. Some idea of the size of the soil population can be obtained, nevertheless, by using the fourteen-day averages mentioned above. In Table VIII. are tabulated the average total numbers of flagellates, and amœbæ for the two periods of the year when the population was at its maximum and minimum respectively. An endeavour has also been made to strike a rough balance sheet as to the amount of protoplasm represented by protozoa and bacteria in a ton of soil. For this purpose it has been assumed that the organisms have a specific gravity of 1·0 and are spheres of diameters, 6μ for the flagellates, 10μ for the amœbæ, and 1μ for the bacteria; and that they are uniformly distributed through the top nine inches of soil. The top nine inches of soil is taken as weighing 1000 tons.
TABLE VIII.
| Maximum Period. | Minimum Period. | |||||
|---|---|---|---|---|---|---|
| No. per Gram. |
Weight in Gram per Gram. |
Weight in Tons per Acre. |
No. per Gram. |
Weight in Gram per Gram. |
Weight in Tons per Acre. |
|
| Flagellates | 770,000 | 0·000087 | 0·087 | 350,000 | 0·000039 | 0·039 |
| Amœbæ | 280,000 | 0·000147 | 0·147 | 150,000 | 0·000078 | 0·078 |
| Bacteria | 40,000,000 | 0·000020 | 0·02 | 22,500,000 | 0·000012 | 0·012 |
It must be remembered that the above figures are minimum ones, as many species of bacteria and protozoa, known to occur in the soil, are not included in the statement owing to their not appearing on the media used for counting purposes.
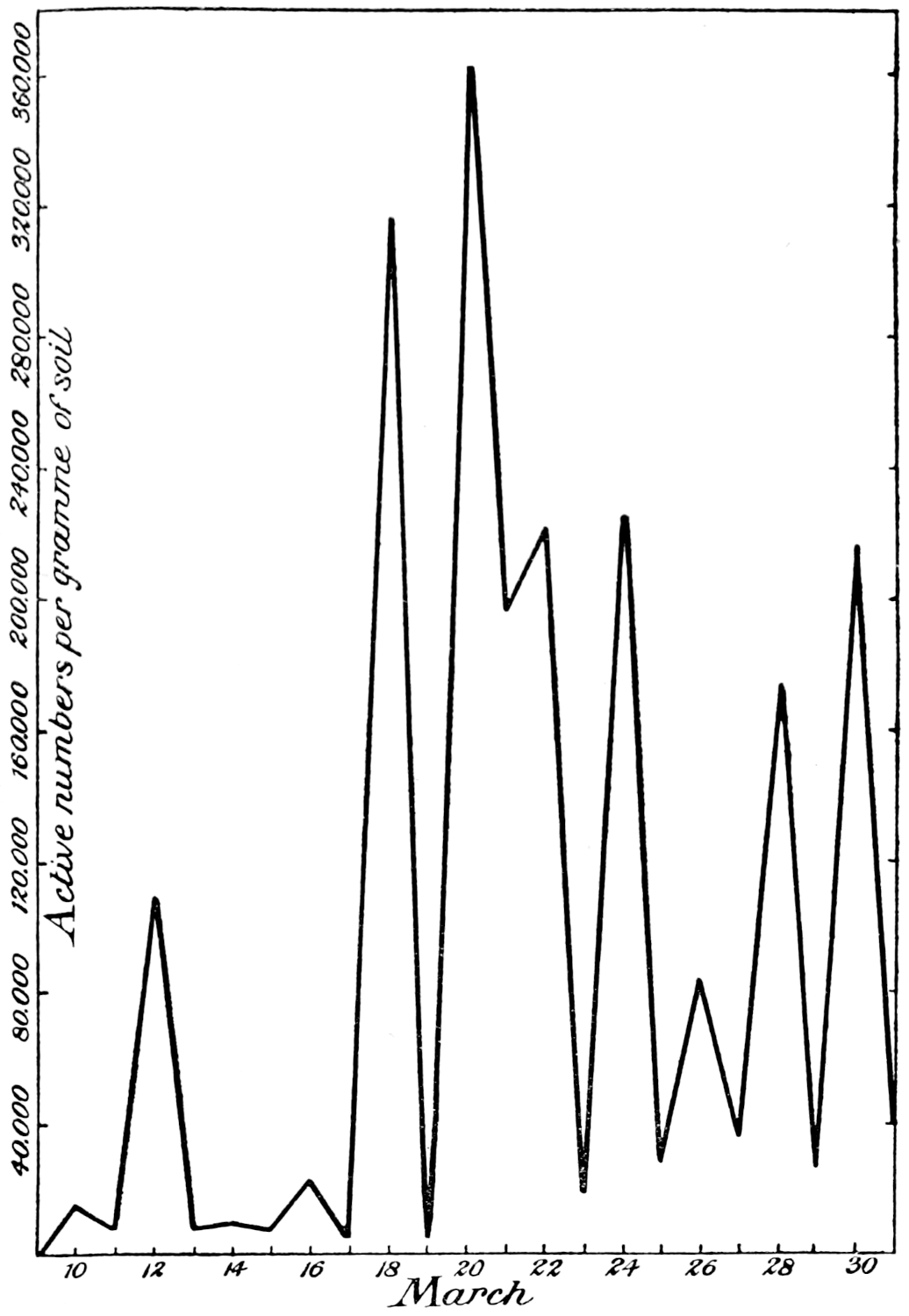
Fig. 16.—Daily variations in the numbers of active individuals of a species of flagellate, Oicomonas termo (Ehrenb.) during March, 1921. (From Phil. Trans. Roy. Soc., vol. ccxi.)
X-axis: March
Y-axis: Active numbers per gramme of soil
Before leaving the discussion of daily variations in numbers of protozoa, reference must be made to the flagellate species. As already mentioned, their active numbers fluctuate rapidly, and for the most part entirely irregularly. One species, however, Oicomonas termo, is characterised by[91] possessing a periodic change; high active numbers on one day being succeeded by low, which are again followed by high on the third day. This rhythm was maintained, with few exceptions, for 365 days (Fig. 16), and has been shown[92] to take place in artificial culture kept under controlled laboratory conditions (Fig. 17).
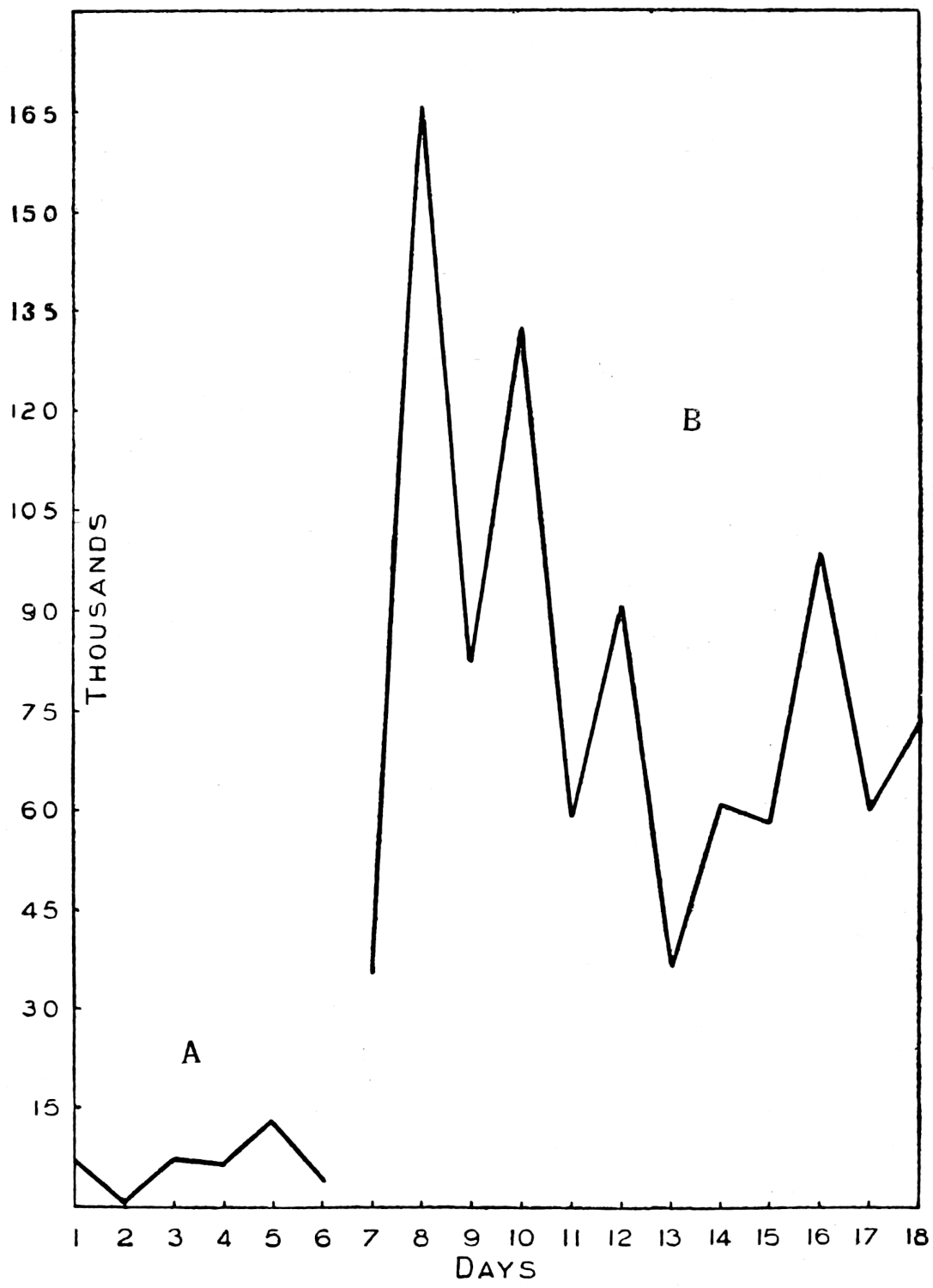
Fig. 17.—Daily variations in the numbers of active individuals of Oicomonas termo (Ehrenb.) in artificial culture media kept at a constant temperature of 20° C. A, in hay infusion; B, in egg albumen.
X-axis: Days
Y-axis: Thousands
[93]
It was thought that an explanation of this phenomenon might be found in alternate excystation and encystation, since the latter is a constituent part of the animals’ life history (see p. 73). This, however, does not hold, for the cyst curve is not the inverse of that of the active; and, moreover, statistical treatment demonstrated that cyst formation is wholly unperiodic in character.
An explanation must therefore be sought in the changes in the organisms during the active period of their life, and the deduction can be drawn that, increased active numbers tend to be followed by death, conjugation, or both, while decreases in the active numbers are followed by rises in total numbers, i.e., reproduction, and this rhythmically.
This somewhat surprising conclusion appears to hold, in a lesser degree, for other soil protozoa, and is of sufficient importance to warrant further research. The direction in which this is being pursued is by a study of the reproductive rates of pure cultures of certain ciliates and flagellates under varying external conditions. Space does not admit of adequate discussion of this problem, but the results already obtained justify the view that such lines of work will elucidate some of the baffling problems of soil micro-biology.
The development of the artificial fertiliser industry has in many ways revolutionised farm practice, with the inevitable result that new problems have arisen, not the least of which are biological in character.
If, as seems to be indubitable, the micro-organisms of the soil are of importance to soil fertility, it is necessary for us to know in what way this population is affected by the application of fertilisers, and a start has been made by investigating the effects of hydrogen ion concentration on soil protozoa. Much has already been written concerning this question, but almost entirely on results obtained in artificial cultures. It is always dangerous to argue from the artificial to the natural environment of organisms and[94] particularly so in respect to the soil. Also, as Collett has shown, the toxic effects of acids are probably not entirely a function of the hydrogen ion concentration, but that the molecules of certain acids are in themselves toxic, an action which can, however, be diminished by the antagonistic powers of many substances such as NaCl.
In this laboratory S. M. Nasir, by unpublished work, has shown that the limiting value on the acid side for Colpoda cucullus was PH 3·3; for a flagellate (Heteromita globosus), 3·5; and for an amœba (Nægleria gruberi), 3·9.
Also Mlle. Perey, investigating the numbers of protozoa in one of the Rothamsted grass plots of PH 3·65, found a total of 13,600 protozoa, of which 90 per cent. were active.
The tolerance, therefore, of these organisms to varying external conditions is greater than has formerly been supposed, a conclusion which is becoming more evident from the researches mentioned in Chapter IV. on soils from different parts of the world.
In partially-sterilised soil from which protozoa were absent Russell and Hutchinson obtained an increased ammonia production, a result also obtained by Cunningham. Hill, on the other hand, concluded that protozoa have no effect on ammonification, but his technique is open to criticism.
Lipman, Blair, Owen and McLean’s work[17] contains many figures obtained by adding dried blood, tankage, soluble blood flour, cottonseed meal, soy-bean meal, wheat flour, corn meal, etc., to soil. It is difficult to understand how accurate results could be expected when, to an already little understood complex substance, such as soil, is added a series of substances whose effects are practically unknown.
Free nitrogen-fixation in soils is an important process, more especially in soils of a light sandy nature, from which crops are taken year after year without any application of manure. The effect of protozoa on the organisms causing this process has in the past received little attention. Recently,[95] however, Nasir[20] has studied the influence of protozoa on Azotobacter, both in artificial culture and in sand. From a total of 36 experiments done in duplicate or triplicate, 31 showed a decided gain in nitrogen fixation over the control, while only 5 gave negative results.
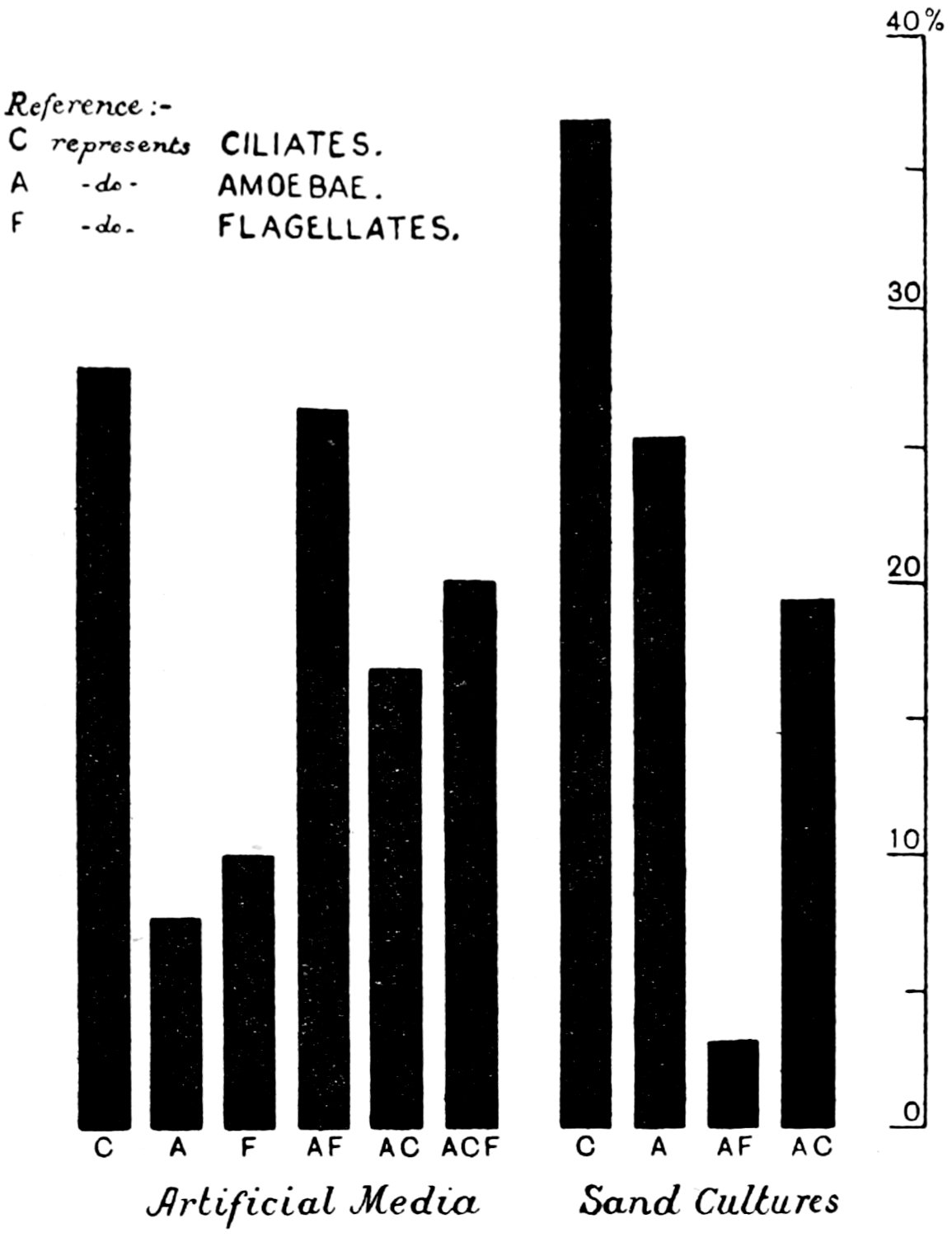
Fig. 18.—Showing the highest fixations of nitrogen above the control recorded for Azotobacter in the presence of different species of Protozoa. (From Ann. Appl. Biol., vol. ii.)
X-axis (left): Artificial Media C A F AF AC ACF
X-axis (right): Sand Cultures C A AF AC
Legend: C represents CILIATES.
A -do.- AMOEBAE.
F -do.- FLAGELLATES.
As might be expected, the fixation figures varied from culture to culture, the highest recorded being 36·04 per cent. above the control and this in a sand culture (Fig. 18).[96] Reference to the details of the experiments shows that the criticisms made against similar work done in the past do not hold here, and one must conclude that Azotobacter is capable of fixing more atmospheric nitrogen in the presence of protozoa than in their absence.
At present it is impossible to say how this occurs, but it is highly improbable that the protozoa are themselves capable of fixing nitrogen. A more likely explanation is that the protozoa, by consuming the Azotobacter, kept down the numbers, and transfer the nitrogen to their own bodies. This will tend to prevent the bacteria from reaching a maximum density, and reproduction, involving high metabolism, will be maintained for a longer period than would have otherwise occurred. This and other possible explanations, are being tested.
Little has been said regarding the application of protozoology to the question of soil partial sterilisation. As already pointed out, in the past much work has been done, but the results were conflicting. In view, however, of our recently acquired knowledge of the life of protozoa in ordinary field soil, most of the early experiments require repeating. A beginning has already been made, but the work is not sufficiently advanced to warrant discussion.
What is urgently needed, however, is to increase our knowledge of the general physiology of these unicellular animals. Until we know what are the inter-relationships between the members of the micro-organic population of normal soil it is almost impossible to hope that means will be devised by which they can be controlled.
At present we are almost entirely ignorant of the simplest of physiological reactions, such as the exact effect of various inorganic salts found in the soil.
Also some experiments in Germany and the States indicate that amœbæ are selective as regards the bacteria they ingest. If this is substantiated it may prove of importance to economic biology.
It has been shown that the flagellates occur in the soil[97] in large numbers, and many of them feed on bacteria. It is probable, however, that certain of them feed saprophytically and must therefore exert some influence on the soil solution, though what this may be is entirely unknown.
Finally, as Nasir has shown, the protozoa play a part in the complicated nitrogen cycle, and work of this type needs extending.
Such, then, are a few of the outstanding problems that confront the soil protozoologist; but he must always remember that the organisms he studies are but a small fraction of the total, and that any influence affecting one part of the complex will be reflected in another. As Prof. Arthur Thomson said in his Gifford Lectures, “No creature lives or dies to itself, there is no insulation. Long nutritive chains often bind a series of organisms together in the very fundamental relation that one kind eats the others.” Such nutritive chains obtain in the soil as markedly as in other haunts of living creatures.
* Papers giving extensive bibliographies.
[1] Cunningham, A., Journ. Agric. Sci., 1915, vol. xvii., p. 49.
[2] Cunningham, A., and Löhnis, F., Centrlb. f. Bakt. Abt. II., 1914, vol. xxxix., p. 596.
[3] Cutler, D. W., Journ. Agric. Sci., 1919, vol. ix., p. 430.
[4] Cutler, D. W., Journ. Agric. Sci., 1920, vol. x., p. 136.
[5] Cutler, D. W., Ann. App. Biol., 1923, vol. x., p. 137.
[6] Cutler, D. W., and Crump, Ann. App. Biol., 1920, vol. vii., p. 11.
[7] * Cutler, D. W., Crump and Sandon, Phil. Trans. Roy. Soc. B., 1922, vol. ccxi., p. 317.
[8] Delf, E. M., New Phytologist, 1915, vol. xiv., p. 63.
[9] Dobell, C. C., Arch. f. Protisenk., 1911, vol. xxiii., p. 269.
[10] * Goodey, T., Roy. Soc. Proc. B., 1916, vol. lxxxix., p. 279.
[11] Goodey, T., Roy. Soc. Proc. B., 1913, vol. lxxxvi, p. 427.
[12] Hartmann, M., and Nägler, K., Sitz-Ber. Gesellsch. Naturf. Freunde, 1908, Berlin, No. 4.
[13] Koch, G. P., Journ. Agric. Res., 1916, vol. li., p. 477.
[14] Kofoid, C. A., Bull. Illinois State Lab. Nat. Hist., 1903 and 1908.
[15] * Kopeloff, N., Lint, H. C., and Coleman, D. A., Centrlb. f. Bakt. Abt. II., 1916, vol. xlvi., p. 28.
[98]
[16] Kopeloff, N., Lint, H. C., and Coleman, D. A., Centrlb. f. Bakt. Abt. II., 1916, vol. xlv., p. 230.
[17] Lipman, J. G., Blair, A. W., Owen, L. L., and McLean, H. C., N.J. Agric. Exp. Sta. 1912, Bull., No. 248.
[18] Martin, C. H. and Lewin, K. R., Journ. Agric. Soc., 1915, vol. vii., p. 106.
[19] Martin, C. H., Roy. Soc. Proc. B., 1912, vol. lxxxv., p. 393.
[20] * Nasir, S. M., Ann. App. Biol., 1923, vol. x., p. 122.
[21] Russell, E. J., Roy. Soc. Proc. B., 1915, vol. lxxxix., p. 76.
[22] * Russell, E. J., “Soil Conditions and Plant Growth,” 1921, 4th ed.
[23] * Sherman, J. M., Journ. Bact., 1916, vol. i., p. 35, and vol. ii., p. 165.
[24] Truffaut, G., and Bezssonoff, H., Compt. Rend. Acad. Sci., 1920, vol. clxx., p. 1278.
[25] West, W., and West, G. S., Journ. Linn. Soc. Bot., 1912, vol. xl., p. 395.
[99]
Speaking broadly, the organisms of the soil may be classified into several distinct groups differing conspicuously in their general characters and physiological functions and therefore in their economic significance; among such groups may be mentioned the bacteria, protozoa, algæ and fungi. It is found, however, that though typical members of these groups are conspicuously different from one another, yet there exist a number of unicellular forms which have characters in common with more than one of these big groups, and the lines of demarcation between them become difficult to define. It becomes advisable, therefore, to depart a little from the systematist’s rigid definitions and to adopt a somewhat more logical grouping of the soil organisms based on their mode of life.
To give but a single example: Euglena viridis occurs quite commonly in soil. Through its single flagellum, its lack of a definite cellulose wall, its changeable shape and its ability to multiply by simple fission in the motile state it definitely belongs systematically to the group of protozoa. But its possession of chlorophyll, in enabling it to synthesise complex organic substances from CO2 and water in a manner entirely typical of plants, connects it physiologically so closely with the lower green algæ that in studying the biology of the soil it seems best to include it and other nearly related forms with the algæ.
On this physiological basis “soil-algæ” may be defined as those micro-organisms of the soil which have the power,[100] under suitable conditions, to produce chlorophyll. Such a definition has the advantage that it is wide enough to include the filamentous protonema of mosses, which, though alga-like in form and in physiological action, is nevertheless separated from the true algæ by a wide gulf. A more accurate name for such a group of organisms would be the “chlorophyll-bearing protophyta” of the soil; they may be classified briefly as follows (Table IX.):—
TABLE IX.
| Group. | Colour. | Pigments. | ||||
|---|---|---|---|---|---|---|
| I. | Flagellatæ. | Euglenaceæ. Cryptomonadineæ. |
Green. | Chlorophyll. | ||
| II. | Algæ— | |||||
| 1. | Myxophyceæ. | Mostly filamentous, chiefly Oscillatoriaceæ and Nostocaceæ. | Blue-green to violet or brown. | Phycocyanin. Chlorophyll. Carotin. |
||
| 2. | Bacillariaceæ. | Mostly pennate, chiefly Naviculoideæ. | Golden-brown. | Carotin. Xanthophyll. Chlorophyll. |
||
| 3. | Chlorophyceæ. | (i) | Protococcales, Ulotrichales, Conjugatæ, etc. | Green. | Chlorophyll. | |
| (ii) | Heterokontæ. | Yellow-green. | Chlorophyll. Xanthophyll. |
|||
| III. | Bryophyta. | Filamentous moss protonema. | Green. | Chlorophyll. | ||
The importance of the lower algæ from a biological standpoint has long been recognised, since their extremely primitive organisation, coupled with their ability to synthesise organic compounds from simple inorganic substances, singles them out as being not very distantly removed from the group of organisms in which life originated upon the earth. But the possibility of their having a very much wider economic significance was completely overlooked until about a quarter of a century ago, when Hensen demonstrated their importance in marine plankton as the producers of the organic substance[101] upon which the whole of the animal life of the ocean is ultimately dependent. In consequence, it has been generally assumed that the growth of algæ, since they contain chlorophyll, is entirely dependent on the action of light. Hence the recent idea of the existence of algæ which actually inhabit the soil has been received with a certain amount of scepticism, though the results of modern physiological research on a number of the lower algæ show that there is very good reason to believe that such a soil flora is entirely possible.
In considering the alga-flora of a soil it is necessary to distinguish between two very different sets of conditions under which the organisms may be growing. In the first place, they may grow on the surface of the soil, being subjected directly to insolation, rain, the deposition of dew, the drying action of wind, relatively quick changes of temperature and other effects of climate. Certain combinations of these conditions present so favourable an environment for the growth of algæ that at times there appears on the surface of the soil a conspicuous green stratum, sometimes so dark in colour as to appear almost black. Strata of this nature are well known, and in systematic works there are constant references to species growing “on damp soil”; for instance, of the 51 well-defined species of Nostoc recognised by Forte, no less than 31 are characterised as terrestrial. Such appearances, however, seem to have been regarded as sporadic and more or less accidental, rather than as the unusually luxuriant development of an endemic population, and have been frequently attributed to an excessively moist condition of the soil due to defective drainage.
In the second place, the algæ may be living within the soil itself, away from the action of sunlight and under somewhat more uniform conditions of moisture and temperature.
Up to the present time the greater number of the investigations carried out in this subject have been of a systematic nature, and extremely little direct evidence has[102] been obtained which can throw any light on the subject of the economic significance of the soil algæ.
The earliest systematic work was carried out by Esmarch, in 1910-11, who investigated by means of cultures the blue-green algæ of a number of soils from the German African Colonies, the samples being taken from the surface and also from the lower layers of the soil. He obtained a considerable number of species and observed that in cultivated soils they were not confined to the surface but occurred regularly to a depth of 10-25 cms. and occasionally as low as 40-50 cms. He attributed their existence in the lower layers to the presence of resting spores carried down in the processes of cultivation, since his samples from uncultivated soils were unproductive.
Later, Esmarch extended his investigations to a far larger number of samples, 395 in all, of soils of different types from Schleswig-Holstein. He found that blue-green algæ were very widely distributed in soils of certain types, though they occurred rarely in uncultivated soils of low water-content, and he described no less than 45 species of which 34 belonged to the Oscillatoriaceæ and Nostocaceæ. Certain of the commoner species were obtained from soils of widely different types, as shown in Table X., while other forms occurred only rarely and with a much more limited distribution.
TABLE X.—FREQUENCY OF OCCURRENCE OF CERTAIN COMMON SPECIES IN ESMARCH’S SOIL SAMPLES.
| Species. | Percentage of Samples containing given Alga. |
|||||
|---|---|---|---|---|---|---|
| Uncultivated Damp Sandy Soil. |
Cultivated Soils. | |||||
| Shores of Elbe. |
Shores of Lakes. |
Sea- shore. |
Sandy. | Clay. | Marsh- land. |
|
| Anabæna variabilis | 46 | 43 | 9 | 10·3 | 60 | 46 |
| Anabæna torulosa | 31 | 14·3 | 63·6 | 27·6 | 34·3 | 56·4 |
| Cylindrospermum muscicola | 23 | 28·6 | 0 | 24 | 48·6 | 59 |
| Cylindrospermum majus | 0 | 14·3 | 0 | 38 | 40 | 33·3 |
| Nostoc Sp. III. | 7·7 | 0 | 0 | 38 | 37 | 48·7 |
[103]
Taking the number of samples containing blue-green algæ as a rough measure of their relative abundance, Esmarch obtained the following interesting figures (Table XI.):—
TABLE XI.
| Kind of Soil. | Percentage of Samples Containing Blue-green Algæ. |
Number of Samples Examined. |
|||
|---|---|---|---|---|---|
| Cultivated marshland | 95 | 40 | |||
| Cultivated clay soil | 94·6 | 37 | |||
| Uncultivated moist sandy soils | 88·6 | 35 | |||
| Cultivated sandy soil | 64·4 | 45 | |||
| Uncultivated | - | Woodland | 12·5 | 40 | |
| Sandy heathland | 9 | 34 | |||
| Moorland | 0 | 35 | |||
In noting that the soils fell into two groups, those relatively rich and those poor in blue-green algæ, Esmarch concluded that the two chief factors governing the distribution of the Cyanophyceæ on the surface of soils are, (1) the moisture content of the soil, (2) the availability of mineral salts, cultivated soils being especially favoured in both of these respects. He further distinguished between cultivated land of two kinds, viz. arable land and grass land, and found that on all types of soil grassland was richer in species than was arable land.
Esmarch examined, in addition, 129 samples taken from the lower layers of the soil immediately beneath certain of his surface samples, 107 at 10-25 cms. and the rest at 30-50 cms. depth.
In cultivated soils, whether grassland or arable land, he found that blue-green algæ occurred almost invariably in the lower layers in those places bearing algæ on the surface and that, with rare exceptions, the algæ found in the lower layers corresponded exactly to those on the surface, except that with increasing depth there was a progressive reduction in the number of species.
[104]
In uncultivated, moist, sandy soils the agreement was far less complete, for though algæ were rarely absent from the lower layers their vertical distribution was frequently disturbed by the action of wind and rain. Other uncultivated soils not subject to periodic disturbance were found to be uniformly lacking in algæ in the lower layers, but as the limited number of samples examined came completely from places where there were no algæ on the surface this means very little.
By direct microscopic examination of soil Esmarch claims to have found living filaments of blue-green algæ at various depths below the surface. He realised, however, that there was no indication of the length of time that such filaments had been buried, and therefore conducted a series of experiments from which he concluded that the period during which the algæ investigated could continue vegetatively in the soil after burial varied with different species from 5-12 weeks, but that during the later part of the period the algæ gradually assumed a yellowish-green colour.
It is unfortunate that Esmarch’s investigations were directed only towards the blue-green algæ since observations made in this country indicate that such a series of records gives but a very incomplete picture of the soil flora as a whole.
Petersen, in his “Danske Aërofile Alger” (1915) added considerably to our knowledge of soil algæ, especially of diatoms. Unfortunately he confined his investigations of the green algæ to forms growing visibly on the surface of the ground. He observed, however, that acid soils possessed a different flora from that commonly found on alkaline or neutral soils, the former being dominated by Mesotænium violascens, Zygnema ericetorum, and 2 spp. of Coccomyxa, while the latter were characterised by Mesotænium macrococcum var., Hormidium, 2 spp., and Vaucheria, 3 spp.
Of diatoms he obtained no less than 24 species and varieties from arable and garden soils, and five characteristic of marshy soils, while from forest soils and dry heathland they[105] appeared to be often absent. He omitted all reference to blue-green algæ.
Meanwhile Robbins, examining a number of Colorado soils that contained unprecedented quantities of nitrate, obtained from them 18 species of blue-green algæ, 2 species of green algæ, and one diatom. Moore and Karrer have demonstrated the existence of a subterranean alga-flora of which Protoderma viride, the most constantly occurring species, was shown to multiply when buried to a depth of one metre.
In this country attention was first called to the subject by Goodey and Hutchinson of Rothamsted who, in examining certain old stored soils for protozoa, obtained also a number of blue-green forms which were submitted to Professor West for identification. This ability of certain algal spores to retain their vitality for a long resting period was so very striking that an investigation was begun at Birmingham in 1915 to ascertain whether other forms were equally resistant. The investigation was carried out on a large number of freshly collected samples of arable and garden soils which were first aseptically air-dried for at least a month and then grown in culture. No less than 20 species or varieties of diatoms, 24 species of blue-green and 20 species of green algæ were obtained from these cultures (Table XII.). In the majority of the samples there was found a central group of algæ, including Hantzschia amphioxys, Trochiscia aspera, Chlorococcum humicola, Bumilleria exilis and rather less frequently Ulothrix subtilis var. variabilis, while moss protonema was universally present. These species were thought to form the basis of an extensive ecological plant formation in which, by the inclusion of other typically terrestrial but less widely distributed species smaller plant-associations were recognised.
In certain of the soils, associations consisting very largely of diatoms were present, and it is to be noted that the majority of the forms that have been described are of exceedingly small size. It is doubtless this characteristic which enables[106] them to withstand the conditions of drought to which the organisms of the soil are liable to be subjected, small organisms having been shown to be better able to resist desiccation than are larger ones. Since the soil diatoms belong to the pennate type, they are further adapted to their mode of life by their power of locomotion, which enables them in times of drought to retire to the moister layers of the soil.
In the soils examined in this work blue-green algæ were less universally present than were diatoms or green algæ, and the species found appeared to be more local in occurrence. There seemed to be, however, an association between the three species, Phormidium tenue, Ph. autumnale, and Plectonema Battersii, at least two of the three species having been found together in no less than 16 of the samples, while all three occurred in 7 of them.
TABLE XII.—ALGÆ IN DESICCATED ENGLISH SOILS. (BRISTOL.)
| Group. | Number of Samples Productive. |
Number of Species. | ||
|---|---|---|---|---|
| Maximum per Sample. |
Average per Sample. |
Total. | ||
| per cent. | ||||
| Diatoms | 95·5 | 9 | 3·7 | 20 |
| Blue-green algæ | 77·3 | 7 | 2·5 | 24 |
| Green algæ | 100 | 7 | 4·3 | 20 |
| Moss protonema | 100 | — | — | — |
| Total | — | 20 | 10·5 | — |
It was generally noticeable that those soils found to be rich in blue-green algæ contained only a few species of diatoms, and vice versa. Diatoms appeared most frequently in soils from old gardens, whereas blue-green algæ were more characteristic of arable soils. The green algæ and moss protonema, on the other hand, were distributed universally.
The majority of green algæ typically found in soils are unicellular, but a few filamentous forms occur. With the exception of Vaucheria spp. these are characterised, however,[107] by an ability to break down in certain circumstances into unicellular or few-celled fragments, in which condition identification is often very difficult.
It was also found by cultural examination of a number of old stored soils from Rothamsted that germination of the resting forms of a number of algæ could take place after an exceedingly long period of quiescence. No less than nine species of blue-green algæ, four species of green algæ, and one species of diatom were obtained from soils that had been stored for periods of about forty years, the species with the greatest power to retain their vitality being Nostoc muscorum and Nodularia Harveyana.
Were it not for the recent advances that have been made in our knowledge of the mode of nutrition of many of the lower algæ, it would be very difficult to account for the widespread occurrence of algæ in the soil, for it is undoubtedly true of some of the more highly evolved algæ that their mode of nutrition is entirely typical of that of green plants in general. The application of bacteriological technique to the algæ, however, by Beijerinck, by Artari, and by Chodat and his pupils, and the introduction of pure-culture methods have led to a study of the physiology of some of the lower algæ, in the hope of getting to understand some of the fundamental problems underlying the nutrition of organisms containing chlorophyll. It is impossible here to do more than mention the names of a few of the more important of those who have worked along these lines, such as Chodat, Artari, Grintzesco, Pringsheim, Kufferath, Nakano, Boresch, Magnus and Schindler, and to condense into a few sentences some of their more important conclusions.
It is now established that although in the light the algæ are able to build up their substance from CO2 and water containing dilute mineral salts, yet in such conditions growth is sometimes very slow, and with some species at[108] any rate it is greatly accelerated by the addition of a small quantity of certain organic compounds. The ability of the lower algæ to use organic food materials varies specifically, quite closely related forms often reacting very differently to the same substance, but there have been shown to be a considerable number of forms which can make use of organic compounds to such an extent that they can grow entirely independently of light. In such cases the nutrition of the organism becomes wholly saprophytic, and the chlorophyll may be completely lost; it has frequently been observed, however, that on suitable nutrient media, even in complete darkness, certain algæ continue to grow and retain their green colour, provided that a sufficient supply of a suitable nitrogenous compound is present.
Chlorella vulgaris, an alga frequently found in soil, has been shown to be extremely plastic in its relations to food substances. Given only a dilute mineral-salts solution as food source, it absorbs CO2 from the air, and grows in sunlight with moderate rapidity. The addition of glucose to the medium in the light greatly increases the rate and amount of growth and the size of the cells, while in the dark the colonies not only remain green but have been shown to develop more vigorously than in full daylight. The organism is also able to use peptone as a source of nitrogen in place of nitrates.
Stichococcus bacillaris and Scenedesmus spp., also occurring in soils, have been shown to be almost equally adaptable, though in these cases the organisms grow more slowly in the dark than on the corresponding medium in the light. Liquefaction of gelatine by the secretion of proteolytic enzymes has been shown to be a further property of certain species, resulting in the formation of amino acids such as glycocoll, phenylalanine, dipeptides, etc. This property is, however, possessed by only a limited number of species and in varying degree.
Up to the present very little work of this kind has been done upon algæ actually taken from the soil, and our knowledge[109] is therefore very scanty. Of the species so far examined all show considerable increase in growth on the addition to the medium of glucose and other sugars, and tend to be partially saprophytic; a few have been shown to liquefy gelatine to some extent.
Servettaz, Von Ubisch, and Robbins have also demonstrated that the protonema of some mosses can make use of certain organic substances, especially the sugars, and grow vigorously in the dark. It has been shown, however, that light is essential for the development of the moss plant.
It was thought at Rothamsted that some light might be thrown upon the activities of the soil-algæ by making counts of the numbers present in samples of soil taken periodically within a circumscribed area. A dilution method similar to that in use in the protozoological laboratory was adopted and applied to samples of arable soil taken from the surface, and at depths of 2, 4, 6 and 12 inches vertically beneath. A considerable number of samples were examined in this way from two plots on Broadbalk wheat-field, viz.: the unmanured plot and that receiving a heavy annual dressing of farmyard manure. The numbers in the unmanured soil were observed to fall far short of those in that containing a large amount of organic matter, while in both plots the numbers varied considerably at different times of the year. The chief species in both plots were identical, and their vertical distribution was fairly uniform, but it was observed that the numbers of individuals varied according to the depth of the sample. The 6th and 12th inch samples contained very few individuals of comparatively few species, but the 4th inch samples yielded numbers that were not significantly less than those in the top inch. The 2nd inch sample was usually much poorer in individuals than either the top or the 4th inch.
It is unfortunate that this method of counting is not really satisfactory for the algæ, chiefly because it takes no account of the blue-green forms. The gelatinous envelope which encloses the filaments of these algæ prevents their[110] breaking up into measurable units. Assuming, as appears to be the case for the two plots investigated, that the blue-green algæ are at least as numerous as the green forms, the total numbers should probably be at least twice as great as those calculated. Taking 100,000 as a rough estimate of the number of algæ per gram of manured soil in a given sample, and assuming the cells to be spherical and of average diameter 10µ, it has been calculated that the volume of algal protoplasm present was at least 3 times that of the bacteria though only one-third of that of the protozoa. This is probably only a minimum figure for this sample.
A soil population of this magnitude can not be without effect on the fertility of the soil. When growing on the surface of the ground exposed to sunlight the algæ must, by photosynthesis, add considerably to the organic matter of the soil, but when they live within the soil itself their nutrition must be wholly saprophytic, and they can be adding nothing either to the energy or to the food-content of the soil. How these organisms fit into the general scheme of life in the soil is at present undetermined, and there is a wide field for research in this direction.
Probably the most important limiting factor in British agriculture is the supply of nitrogen available for the growing crop, and it seems likely that the soil-algæ are intimately connected with this question in several ways.
Periodic efforts have been made during the last half century to establish the fact that a number of the lower organisms, including the green algæ, have the power of fixing atmospheric nitrogen and converting it into compounds which are then available for higher plants. This property has been definitely established for certain bacteria, and rather doubtfully for some of the fungi, but until recently no authentic proof had been produced that algæ by themselves could fix nitrogen. The subject is too wide to be discussed in much detail here.
[111]
Schramm in America, working with pure cultures of algæ, tried for ten years to establish the fact of nitrogen fixation, and failed completely; more recently Wann has extended Schramm’s work, and claims to have proved indisputably that, given media containing nitrates as a source of nitrogen and a small amount of glucose, the seven species of algæ tested by him fixed atmospheric nitrogen to the extent of 4-54 per cent. of the original nitrogen content of the medium. So important a result needed corroboration, and Wann’s experiment, with some slight improvements, was therefore repeated at Rothamsted last summer.
This work has not yet been published, but in the whole series of ninety-six cultures, with four different species, each growing on six different media, there is no evidence that nitrogen fixation has taken place; but there has been a total recovery at the end of the experiment of 98·93 per cent. of the original nitrogen supplied. On the other hand, a flaw has been detected in Wann’s method of analysing those media containing nitrates, sufficiently great to account for the differences he obtained between the initial and final nitrogen content of his cultures. Hence, though one hesitates to say that the algæ are unable, given suitable conditions, to fix atmospheric nitrogen, one must admit that no one has yet proved that they can do so.
It is far more likely, however, that the experiments of Kossowitsch and others throw more light on the relation of soil algæ to nitrogen fixation. They affirm that greater fixation of nitrogen is effected by mixtures of bacteria and certain gelatinous algæ than by nitrogen-fixing bacteria alone, and that the addition of algæ to cultures of bacteria produces a stimulating effect only slightly less than that of sugar. It is probable, therefore, that the algæ, in their gelatinous sheaths, provide easily available carbohydrates from which the bacteria derive the energy essential to their work, and that nitrogen fixation in nature is due to the combined working of a number of different organisms rather than to the individual action of single species.
[112]
Russell and Richards have shown that the rate of loss of nitrogen by leaching from uncropped soils is far less than would be expected from a purely chemical standpoint, and suggest that certain organisms are present in the soil which, by absorbing nitrates and ammonium salts as they are formed, remove them from the soil solution and so help to conserve the nitrogen of the soil. It is probable that the soil algæ act in this manner, though to what extent has not yet been determined.
In warmer countries than our own, especially those with an adequate rainfall, the significance of soil algæ is perhaps more obvious to a casual observer. Treub states that after the complete destruction of the island of Krakatoa by volcanic eruption in 1883, the first colonists to take possession of the island were six species of blue-green algæ, viz., Tolypothrix sp., Anabæna sp., Symploca sp., Lyngbya 3 spp. Three years after the eruption these organisms were observed to form an almost continuous gelatinous and hygroscopic layer over the surface of the cinders and stones constituting the soil, and by their death and decay they rapidly prepared it for the growth of seeds brought to the island by visiting birds. Hence the new flora which soon established itself upon the island can be said to have had its origin in the alga-flora which preceded it. Fritsch has also emphasised the importance of algæ in the colonisation of new ground in Ceylon.
Welwitsch ascribes the characteristic colour from which the “pedras negras” in Angola derive their name to the growth of a thick stratum of Scytonema myochrous, a blue-green alga, which gradually becomes black and completely covers the soil. At the close of the rainy season this gelatinous stratum dries up very slowly, enabling the underlying soil to retain its moisture for a longer period than would otherwise be the case.
[113]
The gelatinous soil algæ are probably very important in this respect, for their slow rate of loss of water is coupled with a capacity for rapid absorption, and they are therefore able to take full advantage of the dew that may be deposited upon them and increase the power of the soil to retain moisture.
In the cultivation of rice the algæ of the paddy field have been found to be of extreme importance. Brizi in Italy has shown that although rice is grown under swamp conditions yet the roots of the rice plant are typical of those of ordinary terrestrial plants and have none of the structural adaptations to aquatic life so characteristic of ordinary marsh plants. Hence the plants are entirely dependent for healthy growth upon an adequate supply of oxygen to their roots from the medium in which they are growing. A serious disease of the rice plant, characterised by the browning and dying off of the leaves, which was thought at first to be due to the attacks of fungi, was found to be the effect of the inadequate aeration of the roots, while the entry of the fungi was shown to be subsequent to the appearance of the physiological disease. The presence of algæ in the swamp water was found to prevent the appearance of this disease, in that they unite with other organisms to form a more or less continuous stratum over the surface of the ground, and add to the gases which accumulate there large quantities of oxygen evolved during photosynthesis. The concentration of dissolved oxygen in the water percolating through the soil is thereby raised to a maximum, and the healthy growth of the crop ensured.
This work has been corroborated by Harrison and Aiyer in India, and a sufficient supply of algæ in the swamp water is now regarded as one of the essentials for the production of a good rice crop.
From what has been said, it appears that, although our[114] knowledge of the soil algæ is extremely limited, and our conception of the part they play is largely based on speculation, yet the subject is one of enormous interest and worthy of investigation in many directions. In its present undeveloped state, it is a little difficult to foresee which lines of study are likely to prove most profitable, but there is little doubt that eventually the soil algæ will be shown to play a significant part in the economy of the soil.
* Papers giving extensive bibliographies.
[1] Bristol, B. M., “On the Retention of Vitality by Algæ from Old Stored Soils,” New Phyt., 1919, xviii., Nos. 3 and 4.
[2] Bristol, B. M., “On the Alga-Flora of some Desiccated English Soils: an Important Factor in Soil Biology,” Annals of Botany, 1920, vol. xxxiv., No. 133.
[3] Brizi, U., “Ricerche sulla Malattia del Riso detta ‘Brusone,’ Sect. IV. Influenza che le alghe verdi esercitano in risaia,” Annuario dell Instituzione Agraria Dott. A. Ponti, Milan, 1905, vol. vi., pp. 84-89.
[4] Esmarch, F., “Beitrag zur Cyanophyceen-Flora unserer Kolonien,” Jahrb. der Hamburgischen wissensch. Anstalten, 1910, xxviii., 3. Beiheft, S. 62-82.
[5] Esmarch, F., “Untersuchungen über die Verbreitung der Cyanophyceen auf und in verschiedenen Boden,” Hedwigia, 1914, Band lv., Heft 4-5.
[6] Fritsch, F. E., “The Rôle of Algal Growth in the Colonisation of New Ground and in the Determination of Scenery,” Geog. Journal, 1907.
[7] Harrison, W. H., and Aiyer, P. A. Subramania, “The Gases of Swamp Rice Soils,” Mem. Dept. Agr. in India, Chem. Ser. (I.) “Their Composition and Relationship to the Crop,” 1913, vol. iii., No. 3; (II.) “Their Utilisation for the Aeration of the Roots of the Crop,” 1914, vol. iv., No. 1; (IV.) “The Source of the Gaseous Soil Nitrogen,” 1916, vol. v., No. 1.
[8a] Hensen, V., “Ueber die Bestimmung des Planktons oder des im Meere treibenden Materials am Pflanzen und Thieren.” Fünfter Ber. Komm. wiss. Unters. deutschen Meere, 1887.
[8] Moore, G. T., and Karrer, J. L., “A Subterranean Alga Flora,” Ann. Miss. Bot. Gard., 1919, vi., pp. 281-307.
[115]
[9] Nadson, G., “Die perforierenden (kalkbohrende) Algen und ihre Bedeutung in der Natur,” Scripta bot. hort. Univ. Imp. Petrop., 1901, Bd. 17.
[10] Petersen, J. B., “Danske Aërofile Alger,” D. Kgl. Danske Vidensk. Selsk. Skrifter, 7 Raekke, Naturv. og mathem., 1915, Bd. xii., 7, Copenhagen.
[11] Robbins, W. W., “Algæ in some Colorado Soils,” Agric. Exp. Sta., Colorado, 1912, Bulletin 184.
[12] Treub, “Notice sur la nouvelle Flora de Krakatau,” Ann. Jard. Bot. Buitenzorg, 1888, vol. vii., pp. 221-223.
[13] Artari, A., “Zur Ernährungsphysiologie der grünen Algen,” Ber. der D. bot. Ges., 1901, Bd. xix., S. 7.
[14] Artari, A., “Zur Physiologie der Chlamydomonaden (Chlam. Ehrenbergii);” (I.) Jahrb. f. Wiss. Bot., 1913, Bd. lii., S. 410; (II.) Ibid., 1914, Bd. liii., S. 527.
[15] Adjarof, M., “Recherches expérimentales sur la Physiologie de quelques Algues vertes,” Université de Genève—Institut Botanique, Prof. R. Chodat—1905, 6 serie, vii. fascicule, Genève.
[16] Beijerinck, M. W., “Berichte über meine Kulturen niederer Algen auf Nährgelatine,” Centr. f. Bakt. u. Paras., 1893, Abt. I., Bd. xiii., S. 368, Jena.
[17] Boresch, K., “Die Färbung von Cyanophyceen und Chlorophyceen in ihrer Abhängigkeit vom Stickstoffgehalt des Substrates,” Jahrbücher für Wiss. Botanik., 1913, lii., pp. 145-85.
[18] Chodat, R., “Étude critique et expérimentale sur le polymorphisme des Algues,” Genève, 1909.
[19] Chodat, R., “La crésol-tyrosinase, réactif des peptides et des polypeptides, des protéides et de la protéolyse,” Archiv. des Sciences physiques et naturelles, 1912.
[20] Chodat, R., “Monographie d’Algues en Culture pure: Matériaux pour la Flore Cryptogamique Suisse,” 1913, vol. iv., fasc. 2, Berne.
[21] Dangeard, P. A., “Observations sur une Algue cultivée à l’obscurité depuis huit ans,” Compt. Rend. Acad. Sci. (Paris), 1921, vol. clxxii., No. 5, pp. 254-60.
[22] Étard et Bouilhac, “Sur la présence de la chlorophyll dans un Nostoc cultivé à l’abri de la lumière,” Compt. Rend., t. cxxvii, 1898.
[23] Grintzesco, J., “Recherches expérimentales sur la morphologie et la physiologie expérimentale de Scenedesmus acutus,” Meyen. Bull. herb. Boiss., 1902, Bd. ii., pp. 219-64 and 406-29.
[24] Grintzesco, J., “Contribution à l’étude des Protococcoidées: Chlorella vulgaris Beyerinck,” Revue générale de Botanique, 1903, xv., pp. 5-19, 67-82.
[116]
[25] * Kufferath, H., “Contribution à la physiologie d’une protococcacée nouvelle, Chlorella luteo-viridis Chod. n. sp. var., lutescens Chod. n. var.,” Recueil de l’institut bot. Léo Errera, 1913, t. ix, p. 113.
[26] Kufferath, H., “Recherches physiologiques sur les algues vertes cultivées en culture pure,” Bull. Soc. Roy. Bot. Belgique, 1921, liv., pp. 49-77.
[27] Magnus, W., and Schindler, B., “Ueber den Einflusz der Nährsalze auf die Färbung der Oscillarien,” Ber. der D. Bot. Gesellschaft, 1912-13, xxx., p. 314.
[28] * Nakano, H., “Untersuchungen über die Entwicklungs- und Ernährungsphysiologie einiger Chlorophyceen,” Journ. College of Sci. Imp. Univ. Tokyo, 1917, vol. xl., Art. 2.
[29] Pringsheim, E., “Kulturversuche mit chlorophyll-führenden Mikroorganismen,” Cohns Beiträge Z. Biol. d. Pflanzen. (I.) Die Kultur von Algen in Agar, 1912, Bd. xi., S. 249; (II.) Zur Physiologie der Euglena gracilis, 1913, Bd. xii., S. 1.; (III.) Zur Physiologie der Schizophyceen, 1913, Bd. xii., S. 99.
[30] Radais, “Sur la culture pure d’une algue verte; formation de chlorophylle à l’obscurité,” Comptes Rendus, 1900, cxxx., p. 793.
[31] Richter, O., “Zur Physiologie der Diatomeen.” (I.) Sitzber. d. kais. Akad. d. W. in Wien, math, naturw. Kl., 1906, Bd. cxv., Abt. I., S. 27; (II.) Denkschrift d. math. naturw. Kl. d. kais. Akad. d. W. in Wien, 1909, Bd. lxxxiv., S. 666; (III.) Sitzber. d. Kais. Akad., etc., 1909, Bd. cxviii., Abt. I., S. 1337.
[32] Richter, O., “Ernährung der Algen,” 1911.
[33] Robbins, W. J., “Direct Assimilation of Organic Carbon by Ceratodon purpureus,” Bot. Gaz., 1918, lxv., pp. 543-51.
[34] Schindler, B., “Ueber den Farbenwechsel der Oscillarien,” Zeitsch. f. Bot., 1913, v., pp. 497-575.
[35] Ternetz, Charlotte, “Beiträge zur Morphologie und Physiologie der Euglena gracilis,” Jahrb. f. Wiss. Bot., 1912, Bd. 51, S. 435.
[36] Berthelot, “Recherches nouvelles sur les microorganismes fixateurs de l’azote,” Comptes Rend., 1893, cxvi., pp. 842-49.
[37] Bouilhac, R., “Sur la fixation de l’azote atmosphérique par l’association des algues et des bactéries,” Comptes Rend., 1896, cxxiii., pp. 828-30.
[38] Bouilhac and Giustiniani, “Sur une culture de sarrasin en présence d’un mélange d’algues et de bactéries,” Comptes Rendus, 1903, cxxxvii., pp. 1274-76.
[39] Charpentier, P. G., “Alimentation azotée d’une algue: Le Cystococcus humicola,” Ann. Inst. Pasteur, 1903, 17, pp. 321-34.
[117]
[40] Fischer, Hugo, “Über Symbiose von Azotobacter mit Oscillarien,” Centr. f. Bakt., 1904, xii.
[41] Frank, B., “Uber den experimentellen Nachweis der Assimilation freien Stickstoffs durch Erdbewohnende Algen,” Ber. der D. Bot. Gesellsch., 1889, vol. vii., pp. 34-42.
[42] Frank, B., “Ueber den gegenwärtigen Stand unserer Kenntnisse der Assimilation elementaren Stickstoffs durch die Pflanze,” Ber. der. D. Bot. Ges., 1889, vii., 234-47.
[43] Frank, B., and Otto, R., “Untersuchungen über Stickstoff Assimilation in der Pflanze,” Ber. der D. Bot. Ges., 1890, viii., 331-342.
[44] Gautier and Drouin, “Recherches sur la fixation de l’azote par le sol et les végétaux,” Compt. Rend., 1888, cvi., pp. 1174-76; General Conclusions, p. 1232.
[45] Kossowitsch, P., “Untersuchungen über die Frage, ob die Algen freien Stickstoff fixiren,” Bot. Zeit., 1894, Heft 5, S. 98-116.
[46] Krüger, W., und Schneidewind, “Sind niedere chlorophyllgrüne Algen imstande, den freien Stickstoff der Atmosphäre zu assimilieren und Boden an Stickstoff zu bereichern?” Landwirtschaftliche Jahrb., 1900, Bd. 29, S. 771-804.
[47] Moore, Benjamin, and T. Arthur Webster, “Studies of the photosynthesis in f.w.a.” (I.) “The fixation of both C and N from atmosphere to form organic tissue by green plant cell”; (II.) “Nutrition and growth produced by high gaseous dilutions of simple organic compounds, such as formaldehyde and methylic alcohol”; (III.) “Nutrition and growth by means of high dilution of CO2 and oxides of N without access to atmosphere,” Proc. Roy. Soc., London, 1920, B. xci., pp. 201-15.
[47a] Moore, B., Whiteley, Webster, T. A., Proc. Roy. Soc., London, B., 1921; xcii., pp. 51-60.
[48] Reinke, J., “Symbiose von Volvox und Azotobacter,” Ber. der d. Bot. Ges., 1903, Bd. xxi., S. 481.
[49] Russell, E. J., and Richards, E. H., “The washing out of Nitrates by Drainage Water from Uncropped and Unmanured Land,” Journ. Agric. Sci., 1920, vol. x., Part I.
[50] Schloesing, fils, and Laurent, E., “Recherches sur la fixation de l’azote libre par les plantes,” Ann. de l’Institut Pasteur, 1892, vi., pp. 65-115.
[51] Schramm, J. R., “The Relation of Certain Grass Green Algæ to Elementary Nitrogen,” Ann. Mo. Bot. Gard., 1914, i., No. 2.
[52] Wann, F. B., “The Fixation of Nitrogen by Green Plants,” Amer. Journ. Bot., 1921, viii., pp. 1-29.
[118]
Note.—I am indebted to my late colleague Miss Sibyl S. Jewson, M.Sc., for permission to include unpublished data from our investigations on the soil fungi.
In 1886 Adametz,[1] investigating the biochemical changes occurring in soils, isolated several species of fungi. It was, however, only with the work of Oudemans and Koning,[17] in 1902 when forty-five species were isolated and described, the majority as new to science, that the real study of the fungus flora of the soil commenced. There is now no doubt that fungi form a large and very important section of the permanent soil population, and certain forms are found only in the soil. Indeed, Takahashi[22] has reversed the earlier ideas by suggesting that fungus spores in the air are derived from soil forms. The majority of investigations on this subject fall, perhaps, into one or more of three classes: (a) purely systematic studies such as those of Oudemans and Koning,[17] Dale,[5] Jensen,[9] Waksman,[25a] Hagem,[8c] Lendner,[12] and others, which consist in the isolation and identification of species from various soils: (b) physiological researches, such as those of Hagem[8c] on the Mucorineæ of Norway, or the many investigations on the biochemical changes in soils produced by fungi, such as those of Muntz and Coudon,[15] McLean and Wilson,[15] Kopeloff,[11] Goddard,[7] McBeth and Scales,[14] and others: (c) quantitative studies, such as those of Remy,[20] Fischer,[6] Ramann,[18] Waksman,[25c] and Takahashi,[22] which involve numerical estimates of the fungus flora in soils.
With very rare exceptions soil fungi cannot be examined in situ, and the necessary basis of any qualitative research is[119] the isolation of the organisms in pure culture. Most soil forms belong to the Fungi imperfecti, and often show considerable plasticity on artificial media. This makes it very difficult to determine them by comparison with type herbarium specimens or published morphological diagnoses. In consequence many soil fungi have not infrequently been given new specific names, as humicola, terricola, and so forth, which is very unsatisfactory, and means that the determinations have little significance.
Furthermore, most artificial media are slight variations on a few common and simple themes, and are very selective, permitting the growth of a moiety only of the fungi present. In addition, many fungi grow so slowly that they are overwhelmed by the more rapidly germinating or spreading forms, or on the other hand, they may be eliminated by the metabolic products of different adjacent colonies. The extremely selective nature of the technique commonly used is shown if one tabulates systematically all the fungi which have been recorded or described in soil investigations. Of Phycomycetes there are fifty-six species of eleven genera; of Ascomycetes twelve species of eight genera; and of Fungi imperfecti, including Actinomycetes but not sterile Mycelia, 197 species of sixty-two genera. Rusts and Smuts one might not expect, but that of the multitudes of Basidiomycetes growing in wood and meadow not one should have been recorded is indeed startling. It was at first thought that many imperfect fungi might be conidial stages of Basidiomycetes, but much search among forms isolated at Rothamsted has, up to the present, failed to reveal clamp connections in the hyphæ.
Since various species of soil fungi have different optimum temperature, humidity and other conditions[3] one would not expect to find an even geographic distribution. Very little is yet known of this aspect, but Rhizopus nigricans, Mucor racemosus, Zygorrhynchus vuilleminii, Aspergillus niger, Trichoderma koningi, Cladosporium herbarum, and many species of Aspergillus, Penicillium, Fusarium, Alternaria, and[120] Cephalosporium have been commonly found throughout North America and Europe wherever soils have been examined. Species of Aspergillus, however, would appear to be more common in the soils of south temperate regions and species of Penicillium, Mucor, Trichoderma, and Fusarium more abundant in northern soils.
It is well known that in many plant and animal communities there occurs a definite rhythm, various species following each other in a regular sequence as dominants in the population. Although it is not yet possible to make any definite statement there would seem indications that this may also be true of the soil fungi.
Much work has been done on the distribution of species at different depths in the soil, but the results are still confusing. Thus, examining eighteen species, Goddard[7] found no difference in relative distribution down to 51⁄2 inches. Werkenthin[26] found identical species from 1-4 inches, and then an absence of fungi from 5-7 inches, which latter was the greatest depth he examined. Waksman[25] found little difference in the first six inches, but very few species below 8 inches except Zygorrhynchus vuilleminii, which extended down to 30 inches and was often the only species occurring below 12 inches. Taylor[23] has reported species of Fusarium at practically every depth to 24 inches. Rathbun[19] found Aspergillus niger, Rhizopus nigricans, and species of Fusarium and Mucor down to 34 inches, and Oospora lactis, Trichoderma koningi, Zygorrhynchus vuilleminii and species of Penicillium, Spicaria and Saccharomyces as deep as 44 inches. Eleven species were isolated from the alimentary canal of grubs and worms, and Rathbun concluded that soil fungi may be spread by these organisms.
On an unmanured grass plot at Rothamsted twenty species were isolated from a depth of 1 inch, nineteen from 6 inches, and eleven from 12 inches, whereas on the unmanured plot of Broadbalk wheat field twenty-six species were obtained from 1 inch, seven from 6 inches, and five from 12 inches. There appeared to be no conspicuous[121] differences between the floras of the two plots save that in the Broadbalk plot there were fewer Mucorales, and Zygorrhynchus mœlleri and Absidia cylindrospora were absent. In the grass plot samples about one-half the forms occurring at the lower levels were isolated also from the upper levels, but in the Broadbalk sample the five forms isolated from 12 inches, and five out of seven of those at 6 inches occurred only at those levels, i.e. each of the three levels appeared to have a specific flora. The difference in depth distribution in these two cases may be due to the fact that in the Broadbalk plot the stiff clay subsoil occurs at 5-7 inches, whereas in the grass plot the depth of soil is greater than 12 inches. Much further work needs to be done on this aspect before any definite conclusion can be reached.
Much scattered information is available concerning the effect of soil type, manuring, treatment, cropping, and so forth upon the fungus content, but no clear issue as yet emerges from the results. Hagem[8] found that cultivated soils vary greatly from forest soils in the species of Mucor present, and that certain species seem to be associated in similar environments. Thus in pinewoods Mucor ramannianus is usually found, together with M. strictus, M. flavus, and M. sylvaticus, and with this “M. Ramannianus Society,” M. racemosus, M. hiemalis, and Absidia orchidis, are frequently associated. The differences found by Hagem between the species of Mucor from forest and cultivated land could not, however, be confirmed by Werkenthin.[26]
Dale,[5] examining sandy, chalky, peaty and black earth soils, found specific differences, although many of the species were common to all. A soil which had been manured continuously for thirty-eight years with ammonium sulphate alone, contained twenty-two species, whereas the same soil with the addition of lime only had thirteen species. Both Goddard[7] and Werkenthin,[26] in their investigations, found a constant and characteristic fungus flora regardless of soil type, tillage, or manuring. Waksman’s[25] studies of[122] forest soils showed few species of Mucor but many of Penicillium and Trichoderma[2]; orchard soil contained no species of Trichoderma, very few of Penicillium, but a large number of species of Mucor; species of Trichoderma were common in acid soils, whilst cultivated garden soil contained all forms. The examination of very differently manured plots on the Broadbalk wheat field at Rothamsted has not shown any striking differences in the fungus flora, all the more important groups of species being represented in every plot, but significant minor differences are present. Thus, plot 13, manured with double ammonium salts, superphosphate and sulphate of potash, is especially rich in “species” of Trichoderma, whereas the unmanured plot contains large numbers of species of green Penicillium, Trichoderma, and a species of Botrytis (pyramidalis?).
The effect of the crop upon the fungus flora is seen in cases where the same crop is grown year after year as in certain flax areas, where species of Fusarium accumulate in the soil and tend to produce “flax sickness.”[13]
As it is not possible to count the soil fungi in situ, any estimation of the numbers present in a soil must be arrived at by indirect means. The method adopted is to make as fine a suspension as possible of a known quantity of soil sample in a known amount of water, dilute this to 1⁄5000, 1⁄10000, and so forth by regular gradations, incubate cubic centimetres of the final dilution on artificial media in petri dishes, and count the colonies of fungi developing in each plate. Using the average figures from a series of duplicate plates, the number of “individual” fungi in a gram of the original soil sample may then be calculated. The very few students who have made quantitative estimations have obtained very unsatisfactory results. In bacterial or protozoal estimations, the shaking of the soil suspension separates the unicellular individuals, so that in the final platings each individual from the soil theoretically gives rise to one[123] colony on the medium. In the case of fungi, the organisms may be in the form of unicellular or multicellular spores or larger or smaller masses of unicellular or multicellular mycelium differing for each particular species or phase of development within the single species. The organisms may be sterile in the soil or form fruiting bodies, consisting of few or myriads of locally or widely distributed spores. In the process of shaking the soil-suspension fungi of different organisation or of differing developmental stages may be broken up and moieties fragmented in totally different ways or to very different degrees. With protozoa and bacteria the relation of soil individual to plate colony is direct; with fungi we do not know what is the soil “individual” nor whether it is the same for different fungi; nor can we yet profitably discuss any significant numerical relationship of plate colonies to soil organisms. Thus Conn[4] has pointed out that the plate count of a fungus indicates only the ability to produce reproductive bodies and found that the spores of one colony of Aspergillus, if distributed evenly through a kilogram of soil, could produce the average plate counts obtained by Waksman. Abundant vegetative growth may, in some species, reduce or inhibit spore formation, so that of two species the one giving a lower count might really be much the more important and plentiful in the soil. Further, the colonies developing in the final plates represent only a selected few of the fungi present in the soil sample, the Basidiomycetes, and no doubt many other forms, being absent. In addition, different media differ among themselves in the average number of colonies developing on the plates, each medium giving, as it were, its own point of view. Thus, in one experiment carried out at Rothamsted by Miss Jewson, using the same soil suspension, twenty plates of Coon’s Agar gave 357 colonies, of Cook’s Agar 246, of Czapek’s Agar 215, and of Prune Agar 366. Thus if one only used Coon’s Agar and Prune Agar one would obtain a total of 723 colonies, whereas the same suspension on Cook’s Agar and Czapek’s Agar would give only 461, and the[124] calculated numbers of fungi per gram of soil would be totally different. Further, if a single medium be taken, it is found that slight alterations in the degree of acidity may make very considerable differences in the final numbers. Thus Coon’s Agar acidified to a hydrogen ion concentration of 5·0 gave as the results of four series the following average numbers of colonies per plate, 17, 23·75, 18, 23. When, however, the medium was acidified to a PH of 4·0 to 4·3, corresponding averages from three series were 38, 46·3, and 44·8; i.e. the final estimations of numbers of fungi in the soil was about twice as great. Again, the degree of dilution of soil suspension used in plating may also be a very serious factor. Thus, if a series of dilutions be made of 1⁄80,000, 1⁄40,000, 1⁄20,000, 1⁄10,000, 1⁄5,000 and 1⁄2,500, the average plate numbers should be in the proportions of 1, 2, 4, 8, 16, and 32 respectively. In an actual experiment, the following average plate numbers were obtained, 15·4, 32·8, 59·1, 104·0, 150, 224·5, which show a very decided reduction in the higher numbers. If, however, dilutions of a suspension of spores of a single species be made, this reduction does not occur.
These are but three of the very numerous factors involved in the technique of quantitative estimation, and every single factor may be the source of errors of similar magnitude, minute fluctuations in the operations leading to the final platings having very considerable effect upon the numbers of colonies that develop.
By critically evaluating each particular factor in the method, and making statistical correction, it has, however, been found possible to obtain series of duplicate plates comparing very favourably and thus to extract certain figures which, whilst not possessing any final value, have yet a certain general and comparative worth. Thus, 20·0, 18·2, and 16·8 were obtained as the averages of six plates each, of a soil suspension divided into three parts, and the individual plate numbers in all three series were within the range of normal distribution. The meaning of these numerical[125] estimates in relation to fungi per gram of soil sample is, however, entirely hypothetical, and to have value quantitative comparison should only be made between single species or groups of species closely related physiologically, and where the technique is standardised.
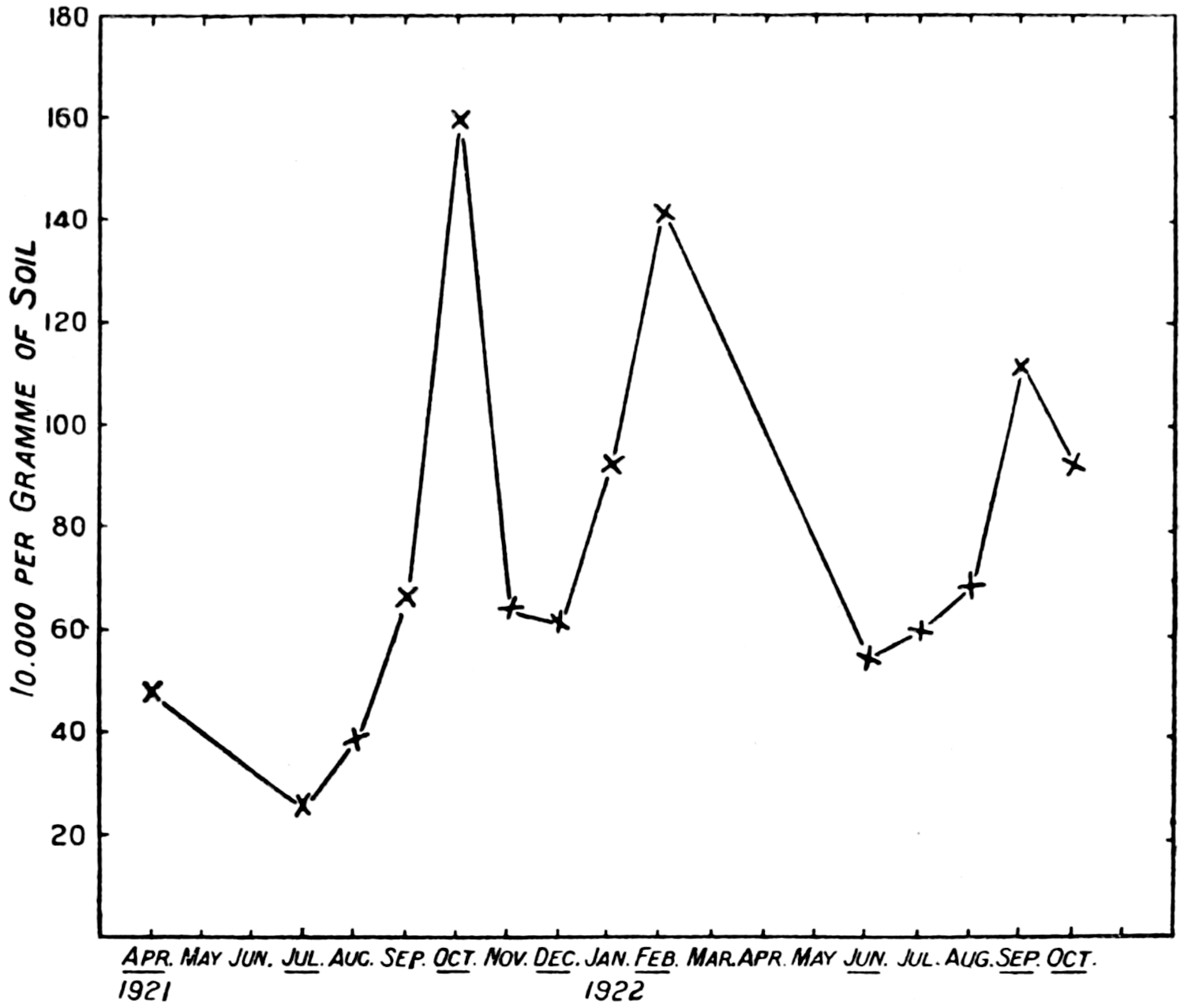
Fig. 19.—Monthly Counts of Numbers of Fungi per gramme of Dry Soil. Broadbalk Plot 2 (Farmyard Manure), Rothamsted.
X-axis: Apr. 1921 May Jun. Jul. Aug. Sep. Oct. Nov. Dec. Jan. 1922 Feb. Mar. Apr. May Jun. Jul. Aug. Sep. Oct.
Y-axis: 10.000 per Gramme of Soil
No comparative estimations have been made of the number of fungi in the soils of different regions. There are, however, certain figures which show that decided seasonal differences exist. Thus, correcting and averaging certain of Waksman’s results[25] the following numbers of fungi per gram of soil at 4 inches deep are obtained; September, 768,000; October, 522,000; November, 310,000; January, 182,000. At Rothamsted results have been obtained which would appear to mark a clear seasonal rhythm,[126] corresponding in the time of its maxima in Autumn and Spring with the periodicities known for many other ecological communities (Fig. 19).
The numbers of fungi at various depths in the soil show very clearly marked differences. The distribution in the top 4-6 inches depending probably upon the depth of soil, is more or less equal, but there is a very rapid falling off in numbers, especially between 5-9 inches, until at 20-30 inches fungi are either very few in number or absent. Thus Takahashi[22] found 590,000 fungi per gram at a depth of 2 cms. and only 160,000 at 8 cms.
TABLE XIII.—INFLUENCE OF SOIL TREATMENT UPON THE NUMBERS OF FUNGI AS DETERMINED BY THE PLATE METHOD—(AFTER WAKSMAN).
| Soil Fertilisation. | Reaction. | Numbers of Fungi per Gram of Soil. |
|---|---|---|
| P.H. | ||
| Minerals only | 5·6 | 37,300 |
| Heavily manured | 5·8 | 73,000 |
| Sodium nitrate | 5·8 | 46,000 |
| Ammonium sulphate | 4·0 | 110,000 |
| Minerals and lime | 6·6 | 26,200 |
| Ammonium sulphate and lime | 6·2 | 39,100 |
The type of soil and its treatment exercise a great influence over the number of fungi present. Fischer[6] found that farmyard manure increased the number of fungi in uncultivated “Hochmoor,” cultivated “Grunlandmoor,” and a clay soil by two, three, and five times respectively. Waksman’s results[25] indicate that the more fertile soils contain more fungi, both in number and species, than the less fertile ones, and if one averages his results, the following figures are obtained: garden soil, 525,000 per gram; orchard soil, 250,000; meadow soil, 750,000; and forest soil, 151,000. Recently Waksman[25e] has found that manure and acid fertilisers increase the numbers of fungi in the soil, whereas the addition of lime decreases them (Table XIII.).
[127]
Jones and Murdock[10] examined surface and sub-surface samples of forty-six soils representing seventeen soil types in eastern Ontario. Molds were fairly uniform in numbers in all soils except a sandy clay loam and sandy clay shale, in which they were absent.
It has also frequently been pointed out that acid and water-logged soils are richer in fungus content than normal agricultural soils. On the other hand, Brown and Halversen[2] found, examining six plots receiving different treatment and studied through a complete year, that the numbers of fungi were unaffected by moisture, temperature, or soil treatment. Against this, however, must be set the work of Coleman[3] who studied the activities of fungi in sterile soils and found such factors as temperature, aeration and food supply to exercise a deciding control.
Investigations at Rothamsted show that Broadbalk plot 13, receiving double ammonium salts, superphosphate and sulphate of potash and yielding 31 bushels per acre, and plot 2, receiving farmyard manure and yielding 35·2 bushels, contain approximately equal numbers of fungi. This figure is about half as high again as that for plot 3, which is unmanured and yields 12·6 bushels, plot 10, with double ammonium salts alone and yielding 20 bushels, and plot 11, with double ammonium salts and superphosphate and yielding 22·9 bushels per acre. A primary factor, however, in all considerations such as these is the equality of distribution of fungi laterally in any particular soil. There are probably few soils so homogeneous as the Broadbalk plots at Rothamsted, and on plot 2 (farmyard manure since 1852) samples taken from the lower and upper ends and the middle region gave average numbers of colonies per plate of 24, 23, and 25 respectively. On the other hand, soil samples taken only a few yards apart in the middle region of the plot gave average plate counts of 33·7 and 56·8.
[128]
Surveying generally the field covered in this chapter, one can only be impressed with the fragmentary character of our knowledge and with the fact that, owing to the selective nature of the technique, the data we possess, if assumed to be representative, give an entirely partial and erroneous picture of the soil fungi. From the qualitative aspect, the chief impediment is the impossibility of obtaining reliable specific determinations of very many of the soil fungi. Lists of doubtfully-named forms from particular soils or geographic regions are useless or a positive evil, and there is imperative need for the systematising of selected genera by physiological criteria, such as has been partially done for Penicillium, Fusarium, and Aspergillus. Furthermore, until a standardised and non-selective technique has been devised, or a number of standardised selective methods for particular groups, comparative investigations into specific distribution can give little of value. This latter criticism is also very applicable if regard be paid to the quantitative aspect of soil work, for progress here largely depends upon the elaboration of a standardised fractionation technique. Every single factor in these methods needs exact analysis, for each gives opportunity for great error, and each error is magnified many thousand times in the final results. Much has been done in this direction at Rothamsted, but more remains to do. Finally, working with single species in sterilised soil under standardised conditions, there is fundamental work to be done on the relation of plate colony to soil “individual.”
[1] Adametz, I., “Untersuchungen über die niederen Pilze der Ackerkrume,” Inaug. Diss., Leipzig, 1886.
[2] Brown, P. E., and Halversen, W. V., “Effect of Seasonal Conditions and Soil Treatment on Bacteria and Molds in Soil,” Iowa Agric. Expt. Sta. 1921, Res. Bull., 56.
[3] Coleman, D. A., “Environmental Factors Influencing the Activity of Soil Fungi,” Soil Sci., 1916, v., 2.
[129]
[4] Conn, H. J., “The Microscopic Study of Bacteria and Fungi in Soil,” N.Y. Agric. Expt. Sta., 1918, Bull. 64.
[5] Dale, E., (a) “On the Fungi of the Soil,” Ann. Mycol., 1912, 10; (b) “On the Fungi of the Soil,” Ann. Mycol., 1914, 12.
[6] Fischer, H., “Bakteriologisch-chemische Untersuchungen; Bakteriologischen Teil,” Landw. Jahrb., 1909, 38.
[7] Goddard, H. M., “Can Fungi living in Agricultural Soil Assimilate Free Nitrogen?” Bot. Gaz., 1913, 56.
[8] Hagem, O., (a) “Untersuchungen über Norwegische Mucorineen I., Vidensk. Selsk, I.,” Math. Naturw. Klasse, 1907, 7; (b) “Untersuchungen über Norwegische Mucorineen II., Vidensk. Selsk. I.,” Math. Naturw. Klasse, 1910, 10.
[9] Jensen, C. N., “Fungus Flora of the Soil,” N.Y. (Cornell) Agric. Expt. Sta., 1912, Bull. 315.
[10] Jones, D. H., and Murdock, F. G., “Quantitative and Qualitative Bacterial Analysis of Soil Samples taken in Fall of 1918,” Soil Sci., 1919, 8.
[11] Kopeloff, N., “The Effect of Soil Reaction on Ammonification by Certain Soil Fungi,” Soil Sci., 1916, 1.
[12] Lendner, A., “Les Mucorinées de la Suisse,” 1908.
[13] Manns, S. F., “Fungi of Flax-sick Soil and Flax Seed,” Thesis, N. Dak. Agric. Expt. Sta., 1903.
[14] McBeth, I. G., and Scales, F. M., “The Destruction of Cellulose by Bacteria and Filamentous Fungi,” U.S. Dept. Agric. Bur. Plant Indust., 1913, Bull. 266.
[15] McLean, H. C., and Wilson, G. W., “Ammonification Studies with Soil Fungi,” N.J. Agric. Expt. Sta., 1914, Bull. 270.
[16] Muntz, A., and Coudon, H., “La fermentation ammoniaque de la terre,” Compt. Rend. Acad. Sci. (Paris), 1893, 116.
[17] Oudemans, A. C., and Koning, C.J., “Prodrome d’une flore mycologique, obtenue par la culture sur gelatin préparée de la terre humeuse du Spanderswoud, près de Bussum,” Arch. Néerland. Sci. Exact et Nat., 1902, s. ii., 7.
[18] Ramann, E., “Bodenkunde,” Berlin, 1905.
[19] Rathbun, A. E., “The Fungus Flora of Pine Seed Beds,” Phytopath., 1918, 8.
[20] Remy, T., “Bodenbakteriologischen Studien,” Centr. f. Bakt., 1902, ii., 8.
[21] Sherbakoff, C. D., “Fusaria of Potatoes,” N.Y. (Cornell) Agric. Expt. Sta., 1915, Mem. 6.
[22] Takahashi, T., “On the Fungus Flora of the Soil,” Anns. Phytopath. Soc., Japan, 1919, 1.
[23] Taylor, M. W., “The Vertical Distribution of Fusarium,” Phytopath., 1917, 7.
[130]
[24] Thom, Ch., “Cultural Studies of Species of Penicillium,” U.S. Dept. Agric. Bur. Animal Indus., 1910, Bull. 118.
[25] Waksman, S. A., (a) “Soil Fungi and their Activities,” Soil Sci., 1916, 2; (b) “Do Fungi Actually Live in the Soil and Produce Mycelium?” Science, 1916, 44; (c) “Is there any Fungus Flora of the Soil?” Soil Sci., 1917, 3; (d) “The Importance of Mold Action in the Soil,” Soil Sci., 1918, 6; (e) “The Growth of Fungi in the Soil,” Soil Sci., 1922, xiv.
[26] Werkenthin, F. C., “Fungus Flora of Texas Soils,” Phytopath., 1916, 6.
[131]
In the last chapter fungi were considered as so many specific but functionless units in the soil. Unless, however, they are regarded merely as inert spore contaminations from the air, a view which is now no longer tenable, their very presence implies the existence of innumerable vital relationships between the organisms and their environment. From this point of view the studies treated in the previous chapter are but the necessary first steps to an understanding of the relation of soil fungi to living plants and of the part played by them in the soil economy.
Older classifications of fungi frequently divided these organisms into four categories—parasites, saprophytes, facultative parasites, and facultative saprophytes, but the further mycological studies are carried the more clearly it is seen that these groups are entirely artificial. There are probably few fungi that cannot, under particular conditions, invade living tissues, and it only seems a question of time before at all events the vast majority of fungi will be grown on synthetic media in the laboratory. From our present point of view the importance of this lies in the fact that fungi living saprophytically in the soil may, given the right conditions or the presence of some particular host plant, become parasites or symbionts, and conversely well-known pathogens may live a saprophytic existence. Thus Cucumber Leaf Spot is caused by Colletotrichum oligochætum, and[132] Bewley[3] has repeatedly isolated this fungus from glasshouse manure and refuse of various kinds. In his early studies, Butler[13] isolated many parasitic species of Pythium from Indian soils, and the presence of P. de Baryanum as a soil saprophyte has been confirmed by Bussey, Peters, and Ulrich.[11] De Bruyn[17] has recently found that most species of Phytophthora, including P. erythroseptica and P. infestans may live as saprophytes in the soil, whilst Pratt[53] has isolated from virgin lands and desert soils various fungi, which cause disease in potatoes. In 1912 Jensen[29] gave a list of twenty-three “facultative parasites” isolated from soil, and these are but a moiety of those which could be listed to-day.
Furthermore, it was shown by Frank[24] many decades ago that forest humus is not merely a mass of the remains of animals and plants, but that a considerable part of its organic substance is made up of fungus hyphæ, which ramify and penetrate in all directions. Evidence is rapidly accumulating that this is also true of most other soils containing organic matter. It is well known that many of the higher plants live in symbiotic or commensal relationship with these humus fungi, which are present in the host tissues as mycorrhiza, and further studies only serve to show the widespread and fundamental nature of this relationship. Thus many Basidiomycetes[50] (species of Tricholoma, Russula, Cortinarius, Boletus, Elaphomyces, etc.) possess a mycorrhizal relationship with various broad leaved trees, such as beech, hazel, and birch[57] and with various conifers and certain Ericales. Other Ericales show this relationship with species of the genus Phoma,[62] many orchids, with species of Rhizoctonia[2] (or Orcheomyces[10]), whilst Gastrodia elata contains Armillaria mellea.[36] Certain species of Pteridophyta and Bryophyta are also known to certain mycorrhizal fungi. Of the numerous fungi taking part in these mycorrhizal relationships, only a small number have yet been identified, but there is little doubt that perhaps the majority of these organisms must be regarded as true soil forms.[14], [45][133] The mycological flora of the soil thus plays an important part in the life of many higher forms of vegetation, and this relationship is a very fruitful field for study.
The great cycle of changes occurring in the soil whereby organic matter is gradually transformed and again made available as plant food is entirely dependent upon micro-organisms. Until a decade ago it was thought that bacteria were by far the most important group concerned in the bringing about of these changes, but recent studies have shown that, in at all events certain arcs of this great organic cycle, the fungi have, perhaps, an equal part to play. The life of fungi in the soil may, for our purposes, be considered from three points of view—their part in the decomposition of carbon compounds, their nitrogen relationships, and their work in the mineral transformations of the soil.
Of primary importance in the carbon relationships of soil fungi is the part played in the decomposition of the celluloses, which compose almost all the structural remains of plant tissues. Our first real knowledge of this subject was given by Van Iterson[28] in 1904 when he showed the wide extent of cellulose destruction by fungi, and devised methods whereby fifteen cellulose-decomposing forms, many of which have since proved to be common soil fungi, were isolated. Three years later Appel[1] published his account of the genus Fusarium, and showed that many of the species could destroy filter paper. A difficulty was introduced in 1908 by Schellenberg,[60] who, working with common soil forms, found that only hemicelluloses and not pure cellulose were destroyed. This has recently been supported by Otto,[48] but from the practical point of view the discussion is academic for the amount of pure cellulose in plants is insignificant.
[134]
In 1913 McBeth and Scales[43] showed that a considerable number of common soil fungi were most active cellulose destroyers, pure precipitated cellulose and cotton being readily attacked. This was supported by McBeth in 1916,[42] whilst Scales[59] has found that most species of Penicillium and Aspergillus decompose cellulose, especially where ammonium sulphate is the source of nitrogen. Waksman[65] tested twenty-two soil fungi and found that eleven decomposed cellulose rapidly and four slowly, whilst Dascewska,[16] Waksman,[66], [67] and others have concluded that soil fungi play a more important part in the decomposition of cellulose and in “humification” than soil bacteria. Schmitz[61] has recently shown that cellulose-destroying bacteria play no important part in the decay of wood under natural conditions.
In addition to the celluloses, practically all simple and complex organic carbon compounds are attacked by soil fungi, and in many cases the decomposition is very rapid.[26] Many Actinomycetes, Aspergilli and Penicillia are active starch splitters, and it is of interest to note that some of the strongest cellulose decomposers (Melanconium sp., Trichoderma sp., and Fusaria) secrete little diastase.[66] The Mucorales apparently do not attack cellulose, but can only utilise pectin bodies, monosaccharides, and partly disaccharides.[26] Dox and Neidig[19] have shown that various species of Aspergillus and Penicillium are able to attack the soil pentosans. Roussy,[58] Kohshi,[24] Verkade and Söhngen,[64] and many other workers have found that fats and fatty acids are readily used as food by soil fungi, and Koch and Oelsner[33] have recently shown that tannins are readily assimilated. Klöcker,[32] Ritter,[56] and others have shown that the utilisation of many carbon compounds is to a large extent determined by the source of nitrogen and its concentration in the pabulum.
There would seem, therefore, no doubt that the decomposition of celluloses and other carbon compounds is of primary importance in the life-activities of soil fungi.
[135]
In this section we shall consider the problems of nitrogen fixation and nitrification, of ammonification, and of the utilisation of nitrogenous compounds by soil fungi.
As soil fungi form so large a part of the soil population, the question of whether they can make use of the free nitrogen of the air is of primary importance. During the last two decades many investigators have attempted to solve the problem, often studying allied or identical species; but if one consults some thirty researches published during this period, opinion is found to be about equally divided. Even, however, in those studies where nitrogen fixation has been recorded the amounts are very slight, usually being below 5 mgrms. per 50 c.c. of solution, and often being obviously within the limits of experimental error. Latham,[37] however, working on Aspergillus niger, recorded variations ranging from a nitrogen loss of 42·5 mgrms. to a nitrogen fixation of 205·1 mgrms. per 50 c.c. of medium. Ternetz[63] found that different strains of Phoma radicis may fix from 2·5 mgrms. of nitrogen in the lowest case, to 15·7 mgrms. in the highest per 50 c.c. of nutrient solution. Duggar and Davis[20] report that Phoma betæ may fix nitrogen in quantities of 7·75 mgrms. per 50 c.c. of medium. The latter authors, in a very able critique of the problem, indicate certain possible sources of error in previous work, and if one examines the studies in which nitrogen fixation has been recorded in the light of these criticisms, it is difficult not to think that, with the exception of the genus Phoma, good evidence for nitrogen fixation by fungi is lacking. Phoma betæ is a common pathogen attacking beets, whilst P. radicis is a mycorrhizal form inhabiting various Ericales. Apart from these exact quantitative studies, which have given a negative verdict, there is a considerable amount of positive but indirect evidence for nitrogen fixation by mycorrhizal fungi,[55] and it is very unfortunate that more of these forms have not been investigated quantitatively. As the[136] evidence stands to-day, one must conclude that the fungus flora does not play any part in the direct nitrogen enrichment of the soil.
Equally obscure is the question of nitrification and denitrification by soil fungi, but this is the result of a lack of study rather than of a plethora of indeterminate researches. Direct nitrification or denitrification has not been established, but the work of Laurent[38] and a few other workers appears to show that soil fungi can reduce nitrates to nitrites.
The second primary nitrogen relationship that we have to consider is the process of ammonification. The ammonifying power of soil fungi was first demonstrated by Muntz and Coudon,[46] and by Marchal[40] in 1893, the former showing that Mucor racemosus and Fusarium Muntzii gave a larger accumulation of ammonia in soil than any of the bacteria tested; and the latter that Aspergillus terricola, Cephalothecium roseum and other soil fungi were active ammonifiers, especially in acid soils. Shibata,[62] Perotti,[49] Hagem,[26] Kappen,[31] Löhnis,[39] and others, have observed that urea, dicyanamide and cyanamide are decomposed with the liberation of ammonia; and Hagem[26] has recorded the same process for peptones, amino acids, and other organic nitrogen compounds in plant and animal remains in the soil. The latter author considers soil fungi more important ammonifying agents in the soil than bacteria, a conclusion in which McLean and Wilson,[44] and perhaps most later workers concur. McLean and Wilson[44] found large differences in the ammonifying powers of various soil fungi, the Moniliaceæ being the strongest ammonifiers, the Aspergillaceæ the weakest. Generic and specific differences have been confirmed by Coleman,[15] Waksman,[67] and other authors. Waksman and Cook[70] suggested that such variations may be due, not to innate differences in the metabolic activities of the several organisms, but to differences in reproductive times, and that there might be some relationship between sporogeny and the ability to accumulate nitrogen. Kopeloff[35] has carried out experiments on the[137] inoculation of sterilised soil with known quantities of spores and found that, although the amount of ammonia accumulated increased with the number of spores the proportion was not direct but modified by the food supply. After the first five days’ growth, the rate of ammonia production varied markedly in a two-day rhythm which seemed to be due to the metabolism of the fungus rather than to recurrent stages of spore formation and germination in the life history. The amount of ammonia liberated has been shown by recent work[66] to depend upon the available sources of carbon and nitrogen. In the absence of a carbohydrate supply the protein is attacked both for carbon and nitrogen, and since more of the former is required much ammonia is liberated. In addition, however, to the carbon and nitrogen control, the process of ammonification by soil fungi is intimately related to physical conditions. Working with pure cultures, McLean and Wilson,[44] Coleman,[15] Kopeloff,[35] Waksman and Cook,[70] and other students, have shown that the amount of ammonia accumulated depends upon such factors as the presence of phosphates, the period of incubation of the fungi, aeration, the moisture in the soil, the temperature, the degree of soil acidity, the type of soil, and so forth.
That fungi take a very important place as ammonifying agents in the soil can no longer be doubted, but the question yet remains to be considered of the balance of profit or loss resulting from their activities. It has usually been considered that a part of the ammonia freed is used by the fungi themselves, but that the greater part is liberated, and so rendered available to nitrifying organisms. Both Neller[47] and Potter and Snyder[51] found that typical soil fungi inoculated into sterile soil grew with a vigour approximately equal to the growth induced by an inoculation of the entire soil flora. This is largely to be accounted for by the fact that when soils are sterilised by heat or by certain chemicals, breaking-down changes occur, and substances are liberated which are peculiarly favourable to fungus growth. This[138] fact must be borne in mind when interpreting ammonification and other studies where the method is that of inoculation of fungi into sterilised soil. In many cases it tends to nullify any application of the results to normal soils, whilst in others the conclusions must be accepted with some reserve. In all cases Potter and Snyder[51] found that fungi caused a diminution in the amount of nitrates, that the ammonia was not much changed in amount, and that there was a decrease in the quantities of soluble non-protein nitrogen. The range of organic and inorganic nitrogenous compounds utilisable by soil fungi is very great. Ritter[56] has shown that certain forms can use the nitrogen of “free” nitric acid in the medium; Ritter,[56] Hagem,[26] and others, that soil fungi can use ammonia nitrogen equally with nitrate nitrogen, and Ehrenberg[21] concluded that soil fungi play a more important part in the building of albuminoids from ammonia than bacteria do. Ehrlich[22] has shown that various heterocyclic nitrogen compounds and alkaloids can serve as sources of nitrogen to soil fungi, whilst Ehrlich and Jacobsen[23] have found that soil fungi can form oxy-acids from amino-acids. Hagem,[26] Povah,[52] Bokorny,[6], [8] and others, state that for many soil forms organic nitrogen sources are better than inorganic sources, and that peptones, amino-acids, urea, and uric acids, etc., are very quickly utilised by species of Mucor, yeasts, and so forth. Butkevitch,[12] and Dox[18] have recently found that it depends on circumstances which compounds of protein molecule can be utilised by particular fungi, and that soil fungi can utilise both amino and amido complexes for the formation of ammonia. In 1919 Boas[4] showed for Aspergillus niger that if a number of nitrogenous compounds are available the fungus absorbs the most highly dissociated.
In the welter of scattered observations on the utilisation of nitrogenous compounds, it is difficult to trace any clear issue. That proteins, amino-acids, and other complex organic compounds are readily broken down to ammonia by soil fungi is clear, and, on the other hand, it is also clear that[139] soil fungi utilise extensively ammonia and nitrates as sources of nitrogen. On which side the balance lies it is yet impossible to say.
Heinze[27] and Hagem[26] have stated that soil fungi make the insoluble calcium, phosphorus, and magnesium compounds in soil soluble and available for plant food; and Butkevitch[12] has used Aspergillus niger in determining the availability of the mineral constituents, but practically no work has yet been carried out on these problems. A further matter on which sound evidence is greatly to be desired is the part played by soil fungi in the oxidation processes of iron and sulphur.
A point which may be mentioned here, as it is of some considerable practical importance, is the large quantity of oxalic, citric, and other acids formed by certain common soil fungi. Acid formation is partly dependent upon the species of fungus—even more the physiological race within the species—and partly upon the substratum, particularly the source of carbon.[5], [54] It is interesting that as a group Actinomycetes do not form acids from the carbon source but alkaline substances from the nitrogen sources.[69]
In the preceding sections an attempt has been made to sketch rapidly the chief outlines of the widespread relationships of soil fungi and of the fundamental part that they play in the biochemical changes occurring in the soil. It will be evident, even from this survey, that their occurrence is of the utmost agricultural importance, both when helpful as in mycorrhizal relationships or as agents in making complex organic materials available as plant food, or when harmful as when causal agents of disease in plants. It is clear that could the soil fungi be controlled to human ends by the encouragement of the useful forms and the elimination of the harmful, a valuable power would be placed in the hands of the grower of plants. Certain aspects of this[140] control, the cruder and more destructive perhaps, are already practicable, whilst the finer and more constructive aspects remain possibilities of to-morrow.
Theoretically, the technique of control is selective in that it aims to determine one or more particular fungi, leaving the remaining flora untouched. Its highest expression is seen, perhaps, in the utilisation of pure cultures of mycorrhizal fungi for horticultural purposes, such as orchid cultivation, but there is no reason why this should not be done for other purposes on a field scale similar to the way in which cultures of special strains of the root nodule organisms of legumes are employed. A second aspect is the direct encouragement of special components of the fungus flora for particular purposes by selective feeding. Thus, in a laboratory experiment, McBeth and Scales[43] record an increase of 2000 times in cellulose-destroying and other soil fungi by this method. It has been pointed out that soil fungus activities such as ammonification, proteolysis and carbohydrate decomposition are controlled by factorial equilibria, and for special purposes it would seem feasible to weight the balance so that particular activities may be favoured. A further step in this direction is the controlling of particular physical conditions so that the activities of certain fungi may be restricted. Professor L. R. Jones[30] and his colleagues at Madison have shown the primary importance of the control of the soil temperature in certain parasitic relationships; the work of Gillespie and Hurst[25] and later workers has demonstrated that the parasitism of certain species and strains of Actinomyces upon the potato is conditioned by definite ranges of soil acidity; and many other relationships of similar nature are known. Data along such lines are rapidly accumulating, and in certain cases are already susceptible of practical application. In other cases, particular soil fungi are less open to persuasive influences, and more drastic treatment needs to be adopted. Certain chemicals mixed intimately with the soil increase or diminish the numbers of particular fungi or groups of[141] fungi; whilst these organisms may be totally eliminated from the soil by wet or dry heat for definite periods or by treatment with potent fungicides such as formaldehyde. Although soil sterilisation and crude treatment in other ways has been practised for decades, the possibility of a more delicate control of soil fungi is only now being realised. Its concrete expression will depend upon the progress that is made in exact knowledge of the activities of soil fungi under natural and controlled conditions, of the balance of factors in the environment which controls any particular function and of the genetic nature of the soil fungi which occur. Each of these aspects is a fruitful field of study.
From a general survey of the researches that have been carried out on soil fungi during the past two decades certain issues emerge. It would seem clear that fungi occupy, perhaps, a primary place as factors in the decomposition of celluloses, and thus may be the chief agents in the transformation of plant remains to humus and to soluble compounds which can be used as food by the nitrogen-fixing bacteria. Furthermore, soil fungi are very important ammonifiers, but whether the balance of ammonia freed is utilised by the fungi themselves, or whether it is made available to nitrifying bacteria is not yet clear. If the latter is the case, soil fungi play a valuable indirect rôle in the accumulation of available plant food in the soil. On the other hand, by utilising nitrates as sources of nitrogen, fungi may play an important part in the depletion of the nitrogenous food in the soil available to crop plants. Thirdly, soil fungi apparently take no part in the direct nitrogen enrichment of the soil. Thus, soil fungi would seem to be the most important factor in the first half of that great cycle whereby organic remains become again available as organic food.
The impression left on one’s mind by the study of the life of fungi in the soil is of an infinitely complex series of moving[142] equilibria, the living activities being determined by both biological and physico-chemical conditions. All these factors play an integral part in the life of the soil fungi and must be considered if a true picture is to be drawn. The principal factors may be classified into the following groups: Most evident, perhaps, are the natures and specificities of the fungi and the relative composition of the fungus flora. Equally important, however, are the quantity and quality of the foods available and the non-biological environment which results from the complex series of physical and chemical changes occurring in the soil causally independent of the organisms present, which interacts with the equally vast series of changes resulting from fungus activities. Finally, one must consider the interacting biological environment of surface animals and plants and the microscopic fauna and flora. The complexities are such that only the application of Baconian principles can unravel them. A beginning has been made in the study of pure cultures of soil fungi on synthetic media, and much valuable data have accrued, but it is obviously not possible to apply directly to soil the results obtained in such work. They remain possibilities; in certain cases probabilities, but nothing more. A further step, one already taken and of great promise, is the investigation of the changes occurring in sterilised soils inoculated with known quantities of one or more pure cultures of particular soil fungi. Such intensive study of single factors in a standardised natural or artificial soil, to which has been added a pedigreed fungus, is, perhaps, the most fruitful avenue of progress. In all such work, however, one must bear acutely in mind the fact that a sterilised soil and, still more, an artificial soil, is a very different complex from a normal soil, and that results obtained from the inoculation of such soils are not applicable directly in the elucidation of ordinary soil processes. At present there is no method known of completely sterilising a soil which does not destroy the original physico-chemical balance. It is evident that the complexities are such that chemist, physicist, and[143] biologist must all co-operate if the significance of the processes is to be understood, and a solid foundation laid for future progress and for practical application.
[1] Appel, O., “Untersuchungen über die Gattung Fusarium,” Mitt. Biol. Reichanst. Land- u. Forstw., 1907, 4.
[2] Bernard, N., “L’évolution dans la symbiose. Les Orchidées et leurs Champignons commensaux,” Ann. Sci. Nat. (Bot.), Ser. 9, 1909, 9.
[3] Bewley, W. F., “Anthracnose of the cucumber under glass,” Journ. Min. Agric., 1922, xxix.
[4] Boas, F., “Die Bildung löslicher Stärke im elektiven Stickstoff-Stoffwechsel,” Ber. deut. bot. Ges., 1919, 37.
[5] Boas, F., und Leberle, H., “Untersuchungen über Säurenbildung bei Pilzen und Hefen II.,” Biochem. Ztschr., 1918, 92.
[6] Bokorny, T., “Benzene derivatives as sources of nourishment,” Zentr. Physiol., 1917, 32.
[7] Bokorny, T., “Sugar fermentation and assimilation,” Allg. Brau. Hopfen Zeit., 1917, 57.
[8] Bokorny, T., “Verhaltung einiger organischer Verbindungen in der lebenden Zelle,” Pflügers Archiv., 1917, 168.
[9] Brown, P. E., “Mould action in soils,” Science, 1917, 46.
[10] Burgeff, H., “Die Wurzelpilze der Orchideen,” Jena. 1909.
[11] Bussey, W., Peters, L., and Ulrich, P., “Ueber das Vorkommen von Wurzelbranderregern im Boden,” Arb. Kais. Biol. Anst. Land- u. Forstw., 1911, 8.
[12] Butkevitch, V. S., “Ammonia as a product of protein transformations caused by mould fungi, and the conditions of its formation,” Recueil d’articles dedié au Prof. C. Timiriazeff, 1916.
[13] Butler, E. J., “An account of the genus Pythium and some Chytridiaceæ,” Mem. Dept. Agr. India, 1907, Bot. Ser. 5, 1.
[14] Christoph, H., “Untersuchungen über die mykotrophen Verhältnisse der Ericales und die Keimung von Pirolaceen,” Beihefte Bot. Centr., 1921, 28.
[15] Coleman, D. A., “Environmental factors influencing the activity of soil fungi,” Soil Sci., 1916, 2.
[16] Dascewska, W., “Étude sur la désagrégation de la cellulose dans la terre de bruyère et la tourbe,” Univ. Genève, Inst. Bot., 1913, S. 8.
[17] De Bruyn, H. L. G., “The saprophytic life of Phytophthora in the soil,” Meded. v. d. Landbouwhoogeschool Wageningen, 1922, xxiv.
[18] Dox, A. W., “Amino acids and micro-organisms,” Proc. Iowa Acad. Sci., 1917, 24.
[144]
[19] Dox, A. W., and Neidig, R. E., “Pentosans in lower fungi,” Journ. Biol. Chem., 1911, 9.
[20] Duggar, B. M., and Davis, A. R., “Studies in the physiology of the fungi. (I.) Nitrogen fixation,” Ann. Mo. Bot. Gard., 1916, 3.
[21] Ehrenberg, P., “Die Bewegung des Ammoniakstickstoffs in der Natur,” Mitt. Landw. Inst., Breslau, 1907, 4.
[22] Ehrlich, F., “Yeasts, moulds, and heterocyclic nitrogen compounds and alkaloids,” Biochem. Ztschr., 1917, 79.
[23] Ehrlich, F., and Jacobsen, K. A., “Über die Umwandlung von Aminosäuren in Oxysäuren durch Schimmelpilze,” Ber. Deut. Chem. Gesell., 1911, 44.
[24] Frank, B., “Ueber die auf Wurzelsymbiose beruhende Ernährung gewisser Bäume durch unterirdische Pilze,” Ber. d. Deut. Bot. Gesell., 1885, 3.
[25] Gillespie, L. J., and Hurst, L. A., “Hydrogen-ion concentration—soil type—common potato scab,” Soil Sci., 1918, 6.
[26] Hagem, O., “Untersuchungen über Norwegische Mucorineen,” Vidensk. Selsk. I., Math. Naturw. Klasse, 1910, 7.
[27] Heinze, B. H., “Sind Pilze imstande den elementaren Stickstoff der Luft zu verarbeiten und den Boden an Gesamtstickstoff anzureichen,” Ann. Mycol., 1906, 4.
[28] Van Iterson, C., “Die Zersetzung von Cellulose durch Aërobe Mikroorganismen,” Centr. f. Bakt., 1904, ii, 11.
[29] Jensen, C. N., “Fungous flora of the soil,” Agric. Expt. Sta. Cornell, Bull. 1912, 315.
[30] Jones, L. R., “Experimental work on the relation of soil temperature to disease in plants,” Trans. Wisc. Acad. Sci., 1922, 20.
[31] Kappen, H., “Ueber die Zersetzung des Cyanamids durch Pilze,” Centr. f. Bakt., 1910, ii, 26.
[32] Klöcker, A., “Contribution à la connaissance de la faculté assimilatrice de douze espèces de levure vis-à-vis de quatre Sucres,” Compt. Rend. Trav. Lab., Carlsberg, 1919, 14.
[33] Koch, A., und Oelsner, A., “Einfluss von Fichtenharz und Tannin auf den Stickstoffhaushalt des Bodens und seiner physikalischen Eigenschaften,” Centr. f. Bakt., 1916, ii, 45.
[34] Kohshi, O., “Ueber die fettzehrenden Wirkungen der Schimmelpilze nebst dem Verhalten des Organfettes gegen Fäulnis,” Biochem. Ztschr., 1911, 31.
[35] Kopeloff, N., “The inoculation and incubation of soil fungi,” Soil Sci., 1916, 1.
[36] Kusano, S., “Gastrodia elata and its symbiotic association with Armillaria mellea,” Journ. Coll. Agric., Imp. Univ., Tokyo, 1911, iv.
[145]
[37] Latham, M. E., “Nitrogen assimilation of Sterigmatocystis niger and the effect of chemical stimulation,” Torrey Bot. Club, Bull. 1909, 36.
[38] Laurent, “Les reduction des nitrates en nitrites par les graines et les tubercles,” Bull. Acad. Roy. Sci. Belg., 1890, 20.
[39] Löhnis, F., “Ammonification of cyanamid,” Ztschr. f. Gärungsphysiol., 1914, v.
[40] Marchal, E., “Sur la production de l’ammoniaque dans le sol par les microbes,” Bull. Acad. Roy. Sci. Belg., 1893, 25.
[41] Mazé, P., Vila et Lemoigne, “Transformation de la cyanamide en urée par les microbes du sol,” Compt. Rend. Acad. Sci., Paris, 1919, 169.
[42] McBeth, I. G., “Studies on the decomposition of cellulose in soils,” Soil Sci., 1916, I.
[43] McBeth, I. G., and Scales, F. M., “The destruction of cellulose by bacteria and filamentous fungi,” U.S. Dept. Agric, Bur. Pl. Ind., 1913, Bull. 266.
[44] McLean, H. C, and Wilson, G. W., “Ammonification studies with soil fungi,” New Jersey Agric. Expt. Sta., 1914, Bull. 270.
[45] Melin, E., “Ueber die mykorrhizenpilze von Pinus silvestris (L.) und Picea abies (L.), Karst.” Svensk. Botan. Tidskr., 1921, xv.
[46] Muntz, A., and Coudon, H., “La fermentation ammoniaque de la terre,” Compt. Rend. Acad. Sci., Paris, 1893, 116.
[47] Neller, J. R., “Studies on the Correlation between the production of carbon dioxide and the accumulation of ammonia by soil organisms,” Soil Sci., 1918, 5.
[48] Otto, H, “Untersuchungen über die Auflösung von Zellulosen und Zellwänden durch Pilze,” Dissert., Berlin, 1916.
[49] Perotti, B., “Uber das physiologische Verhalten des Dicyanamides mit Rücksicht auf seinen Wert als Düngemittel,” Centr. f. Bakt., 1907, ii, 18.
[50] Peyronel, B., “Nuovi casa di rapporti micorizici tra Basidiomiceti e Fanerogame arboree,” Bull. Soc. Bot. Ital., 1922.
[51] Potter, R. S., and Snyder, R. S., “The production of carbon dioxide by moulds inoculated into sterile soil,” Soil Sci., 1918, 5.
[52] Povah, A. H. W., “A critical study of certain species of Mucor,” Bull. Torrey Bot. Club, 1917, 44.
[53] Pratt, O. A., “Soil fungi in relation to diseases of the Irish potato in Southern Idaho,” Journ. Agric. Res., 1918, 13.
[54] Raistrick, H., and Clark, A. B., “On the mechanism of oxalic acid formation by Aspergillus niger,” Biochem. Journ., 1919, 13.
[55] Rayner, M. C., “Nitrogen fixation in Ericaceae,” Bot. Gaz., 1922, 73.
[146]
[56] Ritter, G. E., “Contributions to the physiology of mould fungi,” Voronege, 1916.
[57] Rosseels, E., “L’influence des microorganismes sur la croissance des végétaux supérieurs,” Bull. Soc. Centrale Forest. Belg., 1916, 23.
[58] Roussy, A., “Sur la vie des champignons en milieux Gras,” Compt. Rend. Acad. Sci., Paris, 1909, 149.
[59] Scales, F. M., “The Enzymes of Aspergillus terricola,” Journ. Biol. Chem., 1914, 19.
[60] Schellenberg, H. C., “Untersuchungen über das Verhalten einiger Pilze gegen Hemizellulosen,” Flora, 1908, 98.
[61] Schmitz, H., “The relation of bacteria to cellulose fermentation induced by fungi with special reference to the decay of wood,” Ann. Mo. Bot. Gard., 1919, vi.
[62] Shibata, K., “Uber das Vorkommen vom Amide spaltenden Enzymen bei Pilzen,” Beitr. Chem. Physiol. u. Path., 1904, 5.
[63] Ternetz, C., “Über die Assimilation des atmosphärischen Stickstoffs durch Pilze,” Jahrb. f. wiss. Bot., 1907, 44.
[64] Verkade, P. E., and Söhngen, N. L., “Attackability of cis- and trans-isomeric unsaturated acids by moulds,” Centr. f. Bakt., 1920, ii, 50.
[65] Waksman, S. A., “Soil fungi and their activities,” Soil Sci., 1916, 2.
[66] Waksman, S. A., “The influence of available carbohydrate upon ammonia accumulation by micro-organisms,” Journ. Amer. Chem. Soc., 1917, 39.
[67] Waksman, S. A., “Proteolytic enzymes of soil fungi and Actinomycetes,” Journ. Bact., 1918, 3.
[68] Waksman, S. A., “On the metabolism of Actinomycetes,” Proc. Soc. Amer. Bact. Abstract Bact., 1919, 3.
[69] Waksman, S. A., “The influence of soil reaction upon the growth of Actinomycetes causing potato scab,” Soil Sci., 1922, xiv.
[70] Waksman, S. A., and Cook, R. C., “Incubation studies with soil fungi,” Soil Sci., 1916, 1.
[147]
The micro-organisms of the soil have been fully discussed in the preceding chapters of this volume. There now remains to be considered the fauna of invertebrate animals, other than protozoa, which inhabit that same medium. In the first place, it is necessary to define what groups of invertebrate animals are to be regarded as coming under the category of soil organisms. The latter expression has rather a wide application and, for the present purpose, is held to mean any organism of its kind which, in some stage or stages of its life-cycle, lives on or below the surface of the soil. It will be obvious that, with so comprehensive a definition, the intimacy of the association of these animals with the soil will vary within very wide limits. Some animals pass their whole life-cycle in the soil; others are only present during a limited phase, and not necessarily in a trophic condition, but since their occurrence is constant, they cannot be entirely omitted from consideration.
Unlike the groups of organisms which have been dealt with in the foregoing pages, the invertebrates of the soil do not admit, as a rule, of investigation in culture media. It is, in consequence, much more difficult to achieve in the laboratory the same control over their environmental conditions. This fact in itself largely explains why the interpretations of field observations in animal ecology have not usually been subjected to the test of laboratory experimentation. The study of animal ecology, in so far as the denizens of the soil are concerned, is of very recent birth. It has not,[148] as yet, passed the preliminary stage of cataloguing empirical data, and much spade work will be necessary before the various factors controlling the phenomena actually observed are understood.
Owing to the paucity of information available, this chapter is essentially based upon observations conducted at Rothamsted. Its object is not so much to attempt to evaluate the invertebrate fauna of the soil, as to suggest a line of ecological work demanding investigation on land of many different types.
The method adopted at Rothamsted consists in taking weekly soil samples from a given area for a period of twelve months. Each sample is a cube of soil, with a side dimension of nine inches, and a total content of 729 cubic inches. The samples are taken by means of an apparatus consisting of four iron plates, which are driven into the ground down to the required depth so as to form a kind of box, which encloses a cube of soil (vide Morris, 1922 A). The latter is then removed in layers, each layer being transferred to a separate bag for the purpose. When the complete sample has been extracted, there are five bags containing layers of soil taken from the surface to a depth of 1″, from 1″ to 3″, from 3″ to 5″, from 5″ to 7″, and 7″ to 9″ respectively. Below a depth of 9″ no samples have been taken.
The sample obtained in this manner may be gradually worked into small fragments by hand, and examined whenever necessary under a binocular microscope for the smaller organisms present. This procedure, however, is very tedious and has been replaced by the use of an apparatus consisting of a series of three sieves, with meshes of decreasing size (vide Morris, 1922). The soil is washed through these sieves by means of a stream of water, and the meshes of the final strainer are small enough to retain all except the most minute organisms present, while at the same time they allow the finest soil particles to be carried away.[149] When desirable, the effluent can be passed through a bag or sieve of bolting silk, in order to collect such organisms that may have passed through the third sieve.
In addition to the actual taking and examination of the samples, a botanical survey of the area under investigation is made; chemical and mechanical analyses of the soil are also required. It is further necessary to take soil temperature readings, to determine the moisture content of the samples taken, and the amount of organic matter which they contain.
The various groups of invertebrates represented in the soil may be briefly referred to in zoological order.
Nematoda.—The Nematoda or thread-worms are chiefly animal parasites, nevertheless they usually lead an independent existence in the soil in certain stages of their development. The numerous small species belonging to the family Anguillididæ, or eel-worms, form a definite constituent of the soil fauna; they are generally free-living and non-parasitic. Certain members of this family, however, are enemies of cultivated plants.
Annelida.—Terrestrial Annelida are almost entirely confined to the order Oligochæta, the majority of which are earthworms (Terricolæ), whose whole life-cycle is passed within the confines of the soil. The small white worms of the family Enchytræidæ belong to the aquatic section (Limicolæ) of the order, but they have various representatives which are abundant in damp soil containing organic matter.
Mollusca.—The terrestrial Mollusca are included in the sub-order Pulmonata of the Gastropoda. These organisms, which include the snails (Helicidæ) and slugs (Limacidæ), regularly deposit their eggs in moist earth. Slugs adopt the soil as a frequent habitat, only leaving it for feeding purposes in the presence of sufficient moisture. They are frequent consumers of vegetation, with the exception of Testacella, which is carnivorous.
[150]
Crustacea.—The few species of Crustacea inhabiting the soil belong to the order Isopoda, family Oniscidæ, which are popularly referred to as “woodlice,” “slaters,” etc.
Myriapoda.—The Diplopoda or millipedes include enemies of various crops and are common denizens of the soil. The Chilopoda or centipedes are usually less abundant and are carnivorous. The minute Symphyla are often evident but are of minor importance.
Insecta.—Insects form the dominant element in the invertebrate fauna. Phytophagous species devour the subterranean parts of plants, and notable examples are afforded by the larvæ of Melolontha, Agriotes and Tipula. Saprophagous forms are abundantly represented by the Collembola, and by numerous larval Diptera and Coleoptera. Predaceous species preying upon other members of the soil fauna are exemplified by the Carabidæ and many larval Diptera. Parasitic species pass their larval stages on or within the bodies of other organisms. The groups of Hymenoptera, and the dipterous family Tachinidæ, which exhibit this habit, constitute, along with predaceous forms, one of the most important natural agencies controlling the multiplication of insect life. There are also insects (ants, and other of the aculeate Hymenoptera) which utilize the soil as a suitable medium wherein to construct their habitations or brood chambers, without necessarily deriving their food from the soil. Lastly, there are many insects, notably Lepidoptera, which only resort to the soil for the purpose of undergoing pupation. The insect fauna is, therefore, a closely inter-connected biological complex; for a discussion and an enumeration of its representatives reference may be made to papers by Cameron (1913, 1917), and Morris (1921, 1922 a).
Arachnida.—The two principal classes represented in the soil are the Areinida, or spiders, and the Acarina, or mites, and ticks. The Areinida, which are well-known to be carnivorous, are an unimportant constituent of the fauna. Acarina, on the other hand, are abundant, and exhibit a[151] wide range of feeding habits; most of the soil forms are probably carnivorous, and either free-living or parasitic.
In computing the number of invertebrates normally present in a given type of soil, the method adopted consists of making individual counts of all such organisms as occur in each sample of a series taken over a period of twelve months. This method considerably reduces errors due to season and to the possible deviation of one or more samples from the average. If the total number of these organisms is known for the samples taken, it becomes a simple procedure to arrive at their approximate numbers per acre.
TABLE XIV.
(Based on Morris, 1922 A.)
| Unmanured Plot. |
Manured Plot. |
|
|---|---|---|
| Insects | 2,474,700 | 7,727,300 |
| Larger Nematoda and Oligochæta Limicolæ | 794,600 | 3,600,400 |
| Myriapoda— | ||
| Diplopoda | 596,000 | 1,367,000 |
| Chilopoda | 215,400 | 208,700 |
| Symphyla | 64,000 | 215,500 |
| Total | 875,400 | 1,791,200 |
| Oligochæta (Terricolæ) | 457,900 | 1,010,100 |
| Arachnida— | ||
| Acarina | 215,400 | 531,900 |
| Areinida | 20,200 | 20,200 |
| Total | 235,600 | 552,100 |
| Crustacea (Isopoda) | 33,700 | 80,800 |
| Mollusca (Pulmonata) | 13,500 | 33,700 |
| Total Invertebrata | 4,885,400 | 14,795,600 |
[152]
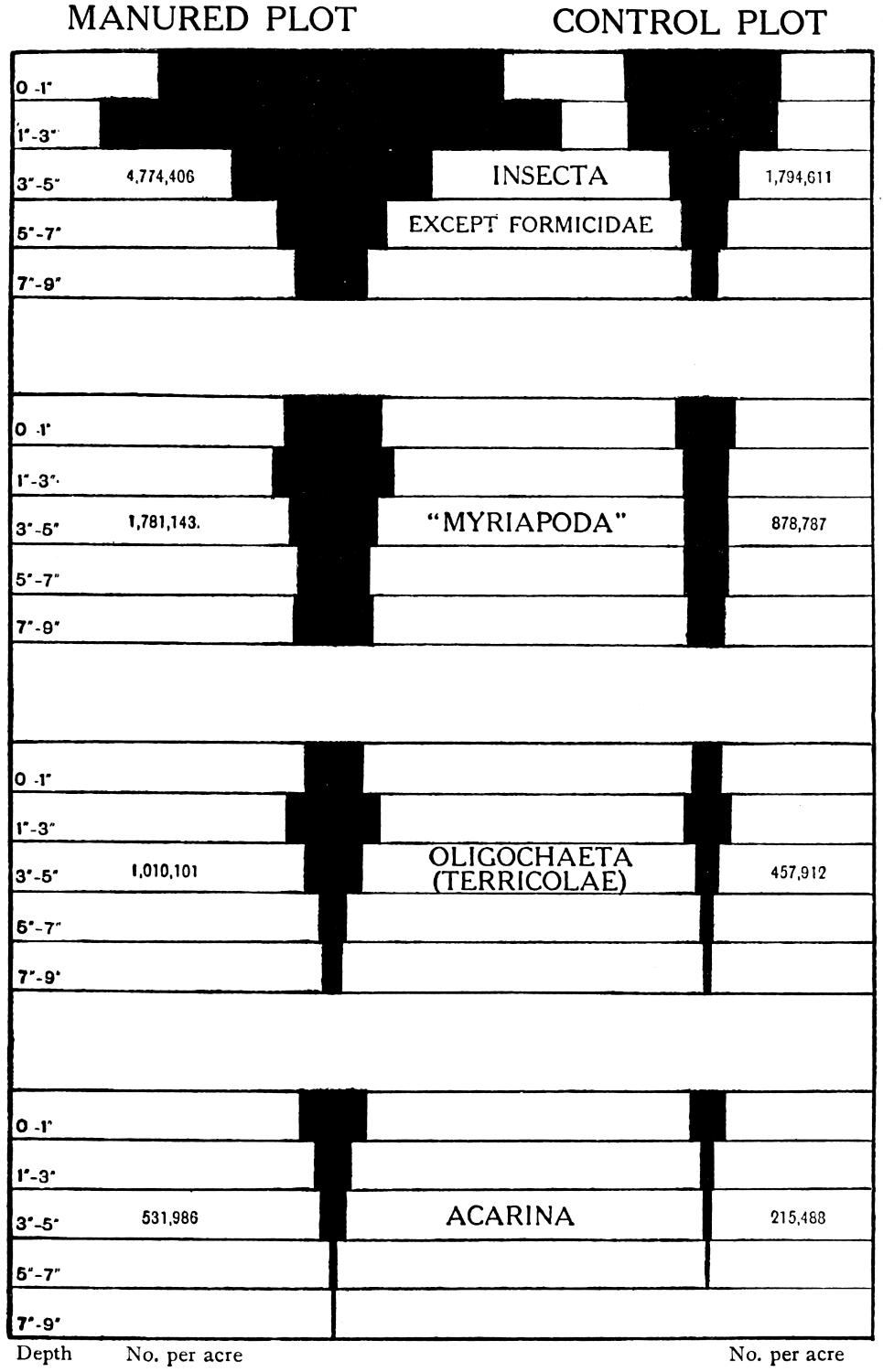
Fig. 20.—Distribution in depth of the more important groups of soil invertebrates in the manured and unmanured (or control) plots at Rothamsted. (From Morris, “Annals of Applied Biology,” vol. ix., nos. 3 and 4, Cambridge University Press.)
[153]
Table XIV. represents a numerical estimate of the invertebrate fauna of two plots of arable land at Rothamsted. The soil is clay with flints overlying chalk, and the land in question has been devoted for eighty years to continuous cropping with wheat; one plot (No. 3) receives an annual dressing of farmyard manure at the rate of 14 tons per acre, and the other plot (No. 2) receives no natural or artificial fertilizer. The significant feature in a comparison of the fauna of the two plots is the great numerical increase in organisms due to the addition of manure. From the point of view of distribution in depth, Fig. 20 clearly demonstrates that the bulk of the fauna is concentrated in the first three inches of the soil. With the exception of the Acarina it is evident that the limits of vertical distribution extend below the depth of nine inches investigated, although the numbers of organisms likely to be present are inconsiderable. The Oligochæta, or true earthworms, occur in Rothamsted soil in numbers very much in excess of the figures given by Darwin, who quoted observations by Hensen. The latter authority calculated that there were 53,767 earthworms in an acre of garden soil, and estimated that about half that number would be present in an acre of corn field. In the Rothamsted investigations their numbers exceeded Hensen’s estimate over 16 times in unmanured land, and over 36 times in manured land.
In an area of pasture-land in Cheshire few insects occurred below a depth of 2 inches, and they reached the limit of their vertical distribution at or near 6 inches. Their number (3,586,000 per acre) is considerably in excess of that present in unmanured arable land at Rothamsted.
[154]

1, Collembola; 2, Thysanura; 3, Orthoptera; 4, Thysanoptera; 5, Hemiptera; 6, Lepidoptera; 7, Coleoptera; 8, Diptera; 9, Hymenoptera.
Fig. 21.—Number of individuals in the different orders of insects in manured and unmanured arable land at Rothamsted. (From Morris, “Annals of Applied Biology,” vol. ix., nos. 3 and 4, Cambridge University Press.)
[155]
In Fig. 21 a numerical analysis is given of the different orders of insects represented in Rothamsted soil. The ascendency of the Hymenoptera and Collembola is almost entirely due to the occurrence of three species in large numbers, viz., the ant Myrmica lævinodis and the Collembola, Onychiurus ambulans and O. fimetarius. In the unmanured plot these two Collembola constituted 13 per cent. of the insects and the species of ant accounted for nearly 28 per cent. In the manured plot they amounted respectively to 27 per cent. and 36 per cent. of the insects present. Next in order of numerical ascendency are larval Diptera, mainly belonging to the families Cecidomyidæ, Chironomidæ, and Mycetophilidæ. The Diptera are followed by the Coleoptera, whose most abundant representatives are larval Elateridæ (wireworms).
1, Collembola; 2, Thysanura; 3, Orthoptera; 4, Thysanoptera; 5, Hemiptera; 6, Lepidoptera; 7, Coleoptera; 8, Diptera; 9, Hymenoptera; 10, Diplopoda; 11, Chilopoda; 12, Areinida; 13, Acarina.
Fig. 22.—Number of species of different orders of invertebrates present in the manured and unmanured (or control) plots at Rothamsted. (From Morris, “Annals of Applied Biology,” vol. ix., nos. 3 and 4, Cambridge University Press.)
[156]
In point of view of number of species present (Fig. 22), Coleoptera take the front rank; in the unmanured plot they are very closely approached by Collembola and Diptera.
Passing from the insects, the next assemblage of organisms in point of number of individuals are the smaller worms. The difficulties attending the specific identification of these organisms are great, and, in the present survey, the Nematodes and all the smaller Oligochætes have not been separated.
The abundance of the Myriapoda is mainly due to the prevalence of Diplopoda, which are represented by four species. The Chilopoda almost entirely consist of a single species Geophilus longicornis.
The dominant group of the Arachnida is the Acarine family Gamascidæ, which are represented by about a dozen species.
| Phyto- phagous. |
Sapro- phagous. |
Carniv- orous. |
Hetero- phagous. |
|
|---|---|---|---|---|
| Unmanured plot | 14 | 48 | 13 | 20 |
| Manured plot | 13 | 58 | 9 | 20 |
From the point of view of the fauna as a whole, the zoological classification of the soil invertebrates is only significant when the various groups are analysed according to the feeding habits of their members. All animals are directly or indirectly dependent upon plant life for their nutrition. For the present purpose they are divided into four categories, and the position of each class of animals in the scheme is based upon the habits of its chief representatives in the soil. Definite information on this subject, however, is not always forthcoming, and it is only possible to achieve approximate estimates. In the table above the[157] percentages in number of individuals present in the two plots investigated at Rothamsted are given under each type of feeding habit.
It must be borne in mind that these estimates only apply to average conditions; the outbreak of a plant pest in any one year must naturally materially alter the proportions given. The phytophagous organisms are represented by a certain number of the Insecta together with the pulmonate Mollusca. Carnivorous forms which are mainly beneficial from the agricultural standpoint, include Insecta, together with the Chilopoda, many Acarina and the Areinida. Saprophagous forms constitute the dominant element of the soil fauna. More than 30 per cent. of the Insecta exhibit this habit, which is also the dominant one in the Oligochæta, Symphyla, and in many of the soil Nematodes. Heterophagous species include all those of somewhat plastic habits; for the most part they are saprophagous, but, on the other hand, a considerable proportion of the species attack growing plants or exhibit both habits. Under this category are included a certain number of the Insecta, the Diplopoda, Isopoda, and some Acarina.
Since animals are endowed with powers of independent locomotion: they are not necessarily tied to their environment to the same extent that plants are. The investigation of the influence of environmental factors sooner or later involves a study of the tropisms of the animals concerned. Until these are adequately understood it is scarcely possible to arrive at any exact conclusions relative to their behaviour in the soil. Insects, for example, respond to the stimuli of various, and often apparently insignificant forces, acting upon their sensory organs. Such responses are known as chemotropism, phototropism, hydrotropism, thermotropism, and so forth according to the nature of the stimuli. Tropisms are automatic and, so far as they distinguish sensations, are[158] independent of any choice, and consequently of psychic phenomena. Animal automatism, however, does not present the rigidity of mechanical automatism. Differential sensibility, vital rhythms, or periodicity, etc., are other important aspects of animal behaviour.
The environmental factors, affecting more especially the insect population of the soil, have been discussed by Cameron (1917) and Hamilton (1917), and certain broader aspects of animal ecology by Adams (1915) and Shelford (1912). These factors are so numerous and so inter-connected, that it is only possible to refer to them briefly in the space available. As might be expected, soils that are of a light and open texture are the ones most frequented by soil insects, nutritional and other factors being equal. Furthermore, it has already been shown that in arable land insects and other animals penetrate to a greater depth than in pastures. This fact is primarily due to the greater looseness of the soil occasioned by agricultural operations, which ensure at the same time better drainage aeration, and greater facilities for penetration. Hamilton found that soil insect larvæ are very sensitive to evaporation, and especially so if the temperature is 20° C. or over. In their natural habitat the relative humidity of the air, in moist or wet soil, is not far below saturation, and the temperature of the soil rarely goes above 20°-23°C., and then only in exposed, dry, hard soil in which these larvæ do not occur.
The significance of the rate of evaporation as an environmental factor was first emphasised by Shelford. According to him the best and more accurate index of the varying physical conditions affecting land animals, wholly or in part exposed to the atmosphere, is the evaporating power of air. By means of a porous cup-atmometer, as devised by Livingston, Shelford has carried out an important series of experiments on the reactions of various animals to atmospheres of different evaporation capacities, and reference should be made to his text-book.
The importance of the organic matter present in the soil[159] is well illustrated in the table on p. 152. The great increase in the number of insects and other animals is partly due to their direct introduction along with the manure, and partly to their entry into the soil in response to chemotropic stimuli exerted by fermentation. Organic matter influences the fauna in other ways also; it increases the moisture content of the soil, and it provides many species with an abundance of food material. Also, the amount of carbon dioxide present in the soil is partly dependent upon decaying organic matter. Hamilton conducted experiments on the behaviour of certain soil insects in relation to varying amounts of carbon dioxide. Although his work is of too limited a nature to be accepted without reserve, it lends support to the conclusions of Adams who says: “The animals which thrive in the soil are likely to be those which tolerate a large amount of carbon dioxide, and are able to use a relatively small amount of oxygen, at least for considerable intervals, as when the soil is wet during prolonged rains. The optimum soil habitat is therefore determined, to a very important degree, by the proper ratio or balance between the amount of available oxygen and the amount of carbon dioxide which can be endured without injury.”
Little is known concerning the occurrence of ammonia in the soil atmosphere, but its presence in minute quantities is probably an important chemotropic factor in relation to saprophagous organisms which are the largest constituent of the fauna. A great increase in Dipterous larvæ occurs on the addition of farmyard manure, and this is noteworthy in the light of Richardson’s experiments (1916), which indicate that ammonia exercises a marked attraction for Diptera, which spend some part of their existence in animal excrement in some form or another.
The nature of the vegetation supported by the soil is of paramount importance in relation to phytophagous organisms, and examples need scarcely be instanced of certain species of soil insects being dependent upon the presence of their specific food plants.
[160]
The relation of these organisms to agriculture may be considered from three points of view: (a) their influence upon the soil itself; (b) their relation to the nitrogen cycle; and (c), their direct influence upon economic plants.
(a) The behaviour of earthworms as a factor inducing soil fertility is discussed by Darwin in his well-known work on the subject, and their action may be briefly summarised as follows. In feeding habits they are very largely saprophagous, and consume decaying vegetable matter including humus, which they swallow, together with large quantities of soil. Earthworms come to the surface to discharge their fæces (“worm casts”), and in this process they are continually bringing up some of the deeper soil to the air. Darwin estimated that earthworms annually brought to the surface of the soil in their “casts” sufficient earth to form a layer ·2 inch in depth, or 10 tons per acre. Their action, along with the atmosphere, are the chief agencies which produce the uniformity and looseness of texture of the surface soil. By means of their burrows earthworms facilitate the penetration of air and water into the soil, while their habit of dragging leaves and other vegetable material into these burrows increases the organic matter present below the surface. These facts are generally agreed upon, but it is a disputed point whether earthworms, by devouring organic matter, aid the conversion of the latter into plant food more rapidly than takes place solely through the activities of micro-organisms.
Soil insects and other arthropods, by their burrowing activities, are also instrumental in loosening the soil texture and thereby facilitating soil aeration and the percolation of water. The action of termites in warmer countries is discussed by Drummond in his “Tropical Africa,” who compares the rôle of subterranean termites to that of earthworms. The great abundance of ants renders them also significant in this same respect, and very few species are direct enemies of the agriculturist.
[161]
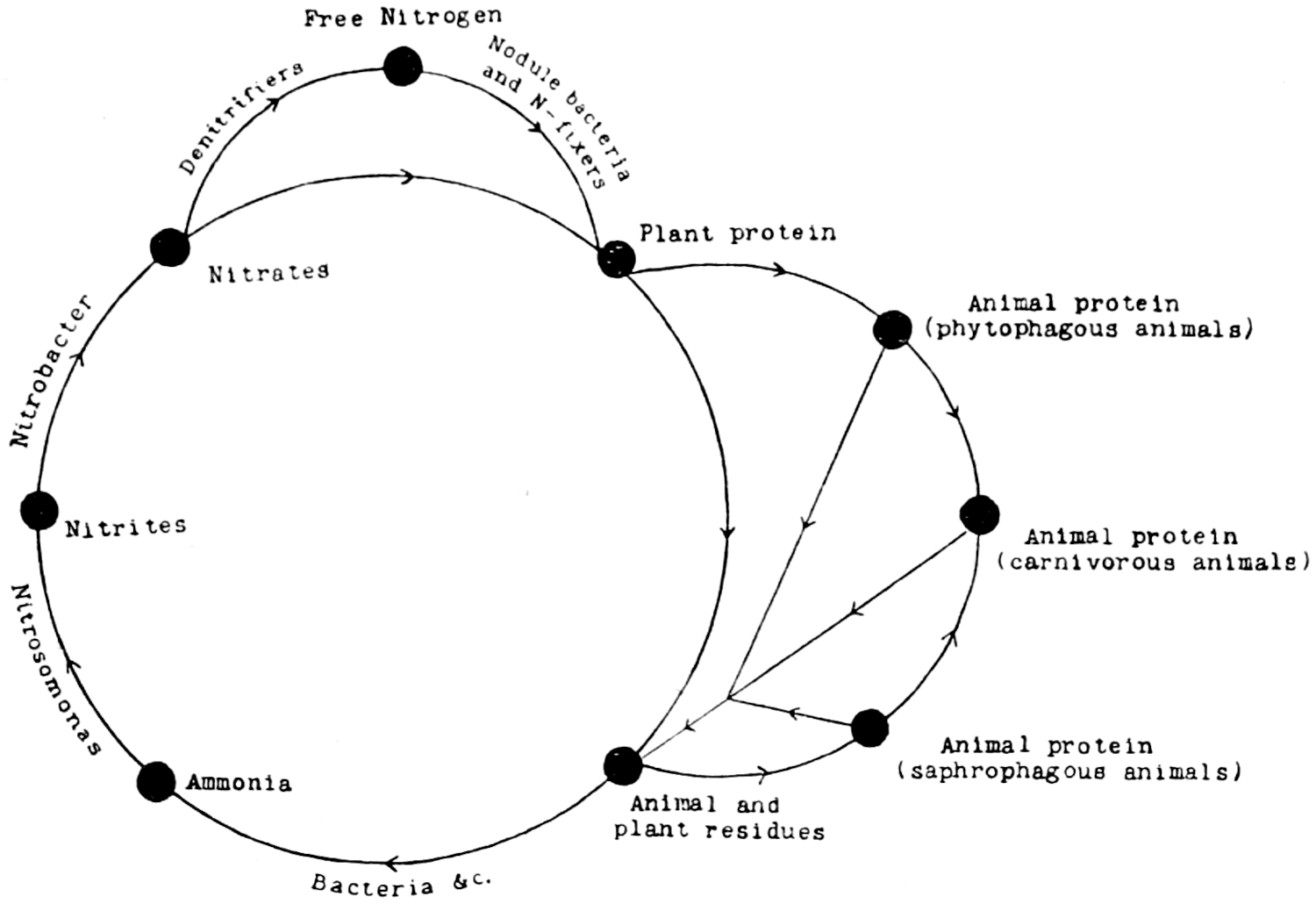
Fig. 23.—Diagram showing the Relation of the Soil Invertebrata (other than Protozoa) to the Nitrogen Cycle.
(b) In their relation to the nitrogen cycle (vide p. 174), the activities of the soil invertebrates may be expressed diagrammatically, as a side-chain in the process (Fig. 23). The proteins, elaborated by plants, are utilised as nitrogenous food by the phytophagous animals present. The waste products of the latter, which contain the nitrogen not used for growth or the replacement of loss by wear and tear, are returned to the soil. Here they disintegrate, and are ultimately converted into ammonium salts, mainly by bacterial action. The dead bodies of these animals are also broken down by various means, becoming eventually chemically dissociated and available as plant food. Animal (and plant) residues serve, however, as food for the large number of saprophagous invertebrates present in the soil. In this event the nitrogen contained in such residues becomes “locked up,” as it were, for the time being in their bodies. Both saprophagous and phytophagous animals are preyed upon by carnivorous species, but ultimately[162] the nitrogen is returned to the soil upon the death of those organisms. The amount present in the bodies of the whole invertebrate fauna has been calculated by Morris (1922) upon analyses furnished by chemists at Rothamsted. It is estimated that the fauna of manured land contains about 7349 grm., or 16·2 lb. of nitrogen per acre, and that of untreated land, 3490 grm., or 7·5 lb. per acre. These amounts are equal respectively to the nitrogen content of 103·6 lb. and 48 lb. of nitrate of soda.
The primary question affecting agriculture is, whether any notable loss of nitrogen is occasioned by the presence of these organisms in the soil. It has been mentioned that their nitrogenous waste material, and their dead bodies, ultimately undergo disintegration; any loss, if any, takes place during the latter process. With the more complex compounds it probably consists in the production of amino-acids and their subsequent hydrolysis or oxidation. During this process an appreciable loss of nitrogen in the gaseous form occurs. This loss, which is discussed on p. 173 would represent the net deficit occasioned by the incidence of invertebrates in the soil. Against this loss must be placed the beneficial action of such organisms as earthworms, which, in all probability, more than counterbalances it.
(c) Many soil insects, on account of their phytophagous habits, are well-known to be some of the most serious enemies of agriculture. Certain of these, and also other classes of invertebrates, which are likewise directly injurious, have been instanced in the earlier pages of this chapter. Detailed information on this subject will be found in textbooks of economic zoology, notably the volume by Reh (1913).
Adams, C. C., “An Ecological Study of Prairie and Forest Invertebrates,” Bull. Illin. St. Lab. Nat. Hist., 1915, xi.
Cameron, A. E., “General Survey of the Insect Fauna of the Soil,” Journ. Econ. Biol., 1913, viii. “Insect Association of a Local Environmental Complex in the District of Holmes Chapel, Cheshire,” Trans. Roy. Soc. Edin., 1917, lii.
[163]
Darwin, C., “Vegetable Mould and Earthworms,” London, 1881.
Hamilton, C. C., “The Behaviour of some Soil Insects in Gradients of Evaporating Power of Air, etc.,” Biol. Bull., 1917, xxxii.
Morris, H. M., “Observations on the Insect Fauna of Permanent Pasture in Cheshire,” Ann. App. Biol., 1921, vii. “On a Method of Separating Insects and other Arthropods from Soil,” Bull. Entom. Res., 1922, xiii. “The Insect and Other Invertebrate Fauna of Arable Land at Rothamsted,” Ann. App. Biol., 1922 A, ix.
Reh, L., In Sorauer’s “Pflanzenkrankheiten,” 1913, iii.
Richardson, C. H., “The Attraction of Diptera to Ammonia,” Ann. Ent. Soc. Amer., 1916, ix.
Russell, E. J., “The Effect of Earthworms on Soil Productiveness,” Journ. Agric. Sci., 1910, iii.
Shelford, V. E., “Animal Communities in Temperate America,” Chicago. 1914, “The Importance of the Measure of Evaporation in Economic Studies of Insects,” Journ. Econ. Entom., 1912, vii.
[164]
In the preceding chapters it is shown that the soil is normally inhabited by a very mixed population of organisms, varying in size from the smallest bacteria up to nematodes and others just visible to the unaided eye, on to larger animals, and finally earthworms, which can be readily seen and handled. These organisms all live in the soil, and therefore must find in it the conditions necessary for their growth. We have dealt in the first chapter with the supplies of water, air, and heat, without which life is clearly impossible. Equally necessary is the source of energy, for the organism requires energy material as surely as the motor engine requires petrol, and it ceases to function unless an adequate supply is forthcoming.
All the energy comes in the first instance from the sun, if we exclude the unknown but probably small fraction coming from radio-active elements. But this radiant energy is not utilisable by the soil population, excepting surface algæ; it has to be transformed into another kind. So far, chlorophyll is the only known transformer; it fixes the energy of sunlight and stores it up in bodies like hemicellulose, sugar, starch, protein, etc. The transformation is imperfect; even the heaviest yielding crops grown under glass, in conditions made as favourable as our knowledge permits, utilise only about 4 per cent. of the total energy available during their period of growth; in natural conditions not more than 0·4 per cent. is utilised. Such as it is, however,[165] the energy fixed in the plant represents all, indeed more than all, that the soil organisms can obtain.
In the state of Nature, vegetation dies and is left on the soil. Two things may then happen. It may become drawn into the soil by earthworms and other agents; the energy supply is thus distributed in the soil to serve the needs of the varied soil population. This is the normal case, associated with the normal soil population and the normal flora. If, however, the mingling agents are absent, the dead vegetation lies like a mat on the surface of the soil, only partially decomposing, unsuitable for the growth of most seedlings, and effectually preventing most of the vegetation below from pushing a way through: thus there comes to be no vegetation at all, or only a very restricted and special flora. The soil population becomes also specialised. Peats and acid grassland afford examples.
On the neutral grass plots at Rothamsted, the dead vegetation does not accumulate on the surface but is rapidly decomposed or drawn into the soil, leaving the surface of the earth bare and free for the growth of seedlings. On the acid plots dead vegetation remains long on the surface, blotting out all new growth excepting two or three grasses which form underground runners capable of penetrating the mat, and sorrel, the seedling roots of which seem to have the power of boring through a fibrous layer of this sort. It is possible to remove the mat entirely by bacterial action alone, if sufficient lime be added periodically to make the reaction neutral, but failing these repeated additions the mat persists.
We shall confine ourselves to the normal case where earthworms bring the source of energy into the soil.
Directly the energy is available, it begins to be utilised. Two laws govern the change. The first is well-known to biologists: it states that the total energy of the system remains constant and can neither be increased nor diminished except from outside; in other words, that energy can be neither created nor destroyed. The second law is less familiar: it is[166] that energy once transformed to heat by one organism cannot be used again by another. It is not destroyed; it remains intact, but is useless to the organism. One cannot have an indefinite chain of organisms living on each other’s excretory products; there was a certain quantity of energy in the food eaten by the first, and no more than this quantity can be got out whether one organism obtains the whole or whether others share it.
The outside value for the amount of energy fixed in the soil is obtainable by combustion of the soil in a calorimeter, but much of this is not available to the soil organisms. The normal sedimentary soils of England still contain decomposition products of the débris of plants and animals originally deposited with them, but in the long course of ages much of the extractable energy has been utilised. The soil population is thus dependent on recently grown vegetation, and it is therefore largely confined to the layer, usually in this country about 6 inches thick, through which the recently dead vegetation is distributed. Below this level there may be sufficient air, water, temperature, etc., but there is insufficient source of energy for any large population.
Unfortunately there is no ready means for distinguishing between the total and the actually available quantity of energy in the soil. But it is not difficult, by adopting the Rothamsted analytical method, to ascertain the approximate amount of energy that has been transformed in a given period. The Rothamsted plots are periodically analysed and a balance sheet is drawn up showing how much of each constituent has been added to and removed from the soil in the intervening period. For two of the Broadbalk plots the results are shown in Tables XV., XVI.
The dunged plot receives 14 tons farmyard manure per annum, a quantity in excess of what would usually be given; the unmanured plot, on the other hand, has received no manure for many years and is abnormally poor. Normal soils lie somewhere between these limits, but tending rather to the value for the dunged than for the unmanured plot. It will be seen that each acre of the dunged land loses on an average 41,000 calories per day, while each acre of the unmanured land loses on an average 2700 calories per day.
[167]
TABLE XV.—MATERIAL BALANCE SHEET: BROADBALK SOIL,
ROTHAMSTED.
(Lb. per Acre per Annum.)
| Farmyard Manure Added. |
No Manure Added. |
|||
|---|---|---|---|---|
| C. | N. | C. | N. | |
| Added in farmyard manure | 3600 | 200 | nil | nil |
| Added in stubble | 300 | 3 | 100 | 1 |
| Total added | 3900 | 203 | 100 | 1 |
| Taken from soil | nil | nil | 200 | nil |
| Stored in soil | 200 | 30 | nil | nil |
| Lost from soil | 3700 | 170 | 300 | nil[H] |
| Per cent. | 95 | 84 | 100 | nil |
| Initial C : N ratio in farmyard manure, 18 : 1 | ||||
| Final C : N ratio in soil, 10 : 1. | ||||
| [H] Gain of 6 lb. See p. 173. | ||||
TABLE XVI.—ANNUAL ENERGY CHANGES IN SOIL: BROADBALK.
APPROXIMATE VALUES ONLY.
Millions of Kilo Calories per Acre per Annum.
| Farmyard Manure Added. |
No Manure Added. |
|||
|---|---|---|---|---|
| Added in manure | 14 | nil | ||
| Added in stubble | 2 | 0·3 | ||
| Total added | 16 | 0·3 | ||
| Taken from soil | nil | 0·5 | -1 | |
| Stored in soil | 0·5 | -1 | nil | |
| Dissipated per annum | 15 | 1 | ||
| Per day: calories | 41,000 | 2700 | ||
| Equivalent to | 12 men. | 3⁄4 man. | ||
| The human food grown provides for | 2 men. | 1⁄2 man. | ||
These numbers are interesting when we reflect that the human food produced on the dunged land yields only 7000[168] calories per day, from which it is clear that our agricultural efforts so far provide more energy for the soil population, for which it was not intended, than for ourselves.
The account is not complete; we have omitted all reference to the oxidation of ammonia and of elements other than carbon. Nature seems to be in an unexpectedly economical mood in the soil, and all compounds which can be oxidised with liberation of energy seem to have corresponding organisms capable of utilising them. Even phenol, benzene, hydrogen, and marsh gas can all be oxidised and utilised as energy sources by some of the soil population.
Even with this remarkable power the soil population has insufficient energy to satisfy all its possibilities; our present knowledge indicates that energy supply is, in this country at any rate, the factor limiting the numbers of the population. Increases in the water supply or the temperature of the soil produce no consistent effect on the population, but directly the energy supply is increased the numbers at once rise.
These transformations of energy involve transformations of matter. The original plant residues may be divided roughly into substances forming the structure of the plant, such as the hemicelluloses, the pentosans, gums, and the contents of the cell—the protoplasm and the storage products, protein; in addition, there are smaller quantities of fats and waxes and other constituents. Some of the easily-decomposable carbohydrates never reach the soil at all, being broken down by intracellular respiration or attack of micro-organisms. But much of the structure material—hemicelluloses, pentosans, etc.—remains.
Once the plant residues pass through the earthworm bodies they become completely disintegrated and lose all signs of structure.
The only visible product so far known is humus, the black sticky substance characteristic of soil and of manure.[169] Two modes of formation have been suggested. Carbohydrates, sugars, pentosans, etc., are known to yield furfuraldehyde or hydroxymethylfurfuraldehyde on decomposition, and it has been shown at Rothamsted that this readily condenses to form a humus-like body, if not humus itself. In the laboratory the reaction is effected in presence of acid, but even amino-acids suffice. All the necessary conditions occur in the soil, and humus formation may proceed in this way.
Some of the structure material—the lignin—contains aromatic ring groupings. Fischer and Schrader have shown that in alkaline conditions these ring substances absorb oxygen and form something very like humus. It is quite possible that humus formation also proceeds in the soil in this way. Whether the two products are chemically identical is not known.
The scheme can be represented thus:—

| Cell structure material | ||
| Aliphatic (Hemicelluloses, Pentosans, etc.) | Aromatic (Lignin, etc., in presence of oxygen and under aerobic conditions) | |
| Fatty acids | Furfuraldehyde or Hydroxymethylfurfuraldehyde (in presence of acid) | |
| Calcium carbonate. | Humus. | |
The disintegration of the cell and the first stages in the decomposition of the structure material are almost certainly brought about by micro-organisms. Whether they complete the process is not known: purely chemical agencies could easily account for part.
The decomposition of protein in the soil has not been[170] studied in any detail. From what is known of the acid hydrolysis and the putrefactive decompositions, however, it is not difficult to draw up a scheme which, at any rate, accords with the facts at present known. It is probable that the protein gives rise to amino-acids, which then break down by one of the known general reactions.
Two types of non-nitrogenous products may be expected: The aliphatic amino-acids give rise to ammonia and fatty acids; these form calcium salts which break down to calcium carbonate. The aromatic amino-acids—tyrosin, phenylalanine, etc.—which would account for about 6 per cent. of the nitrogen of vegetable proteins, would be expected to give ammonia and phenolic substances. Now phenols are poisonous to plants and if no method existed for their removal the accumulation would ultimately render the soil sterile. Matters would be even worse on cultivated soils, since cows’ urine, which enters into the composition of farmyard manure and is the chief constituent of liquid manure, contains, according to Mooser, no less than 0·25 to 0·77 grams of p-cresol per litre,[I] a quantity three to ten times that present in human urine. Fortunately this contingency never arises, for the soil contains a remarkable set of organisms capable of decomposing the phenols and leaving the soil entirely suitable for plant growth. This affords an interesting case of an organism—in this case the plant—growing well in a medium in spite of some adverse condition, not because it is specially adapted to meet this condition, but because some wholly different agent removes it.
[I] Mooser, Zeitschrift physiol. Chem., 1909, lxiii., 176. No phenol was found. It is possible that the p-cresol is not entirely derived from the protein, but that some comes from the glucosides in the animals’ food.
Other ring compounds, e.g. pyrrol, arise in smaller quantity in the decomposition of protein, but their fate in the soil is not known.
We may summarise the probable changes of the protein as follows:—
[171]
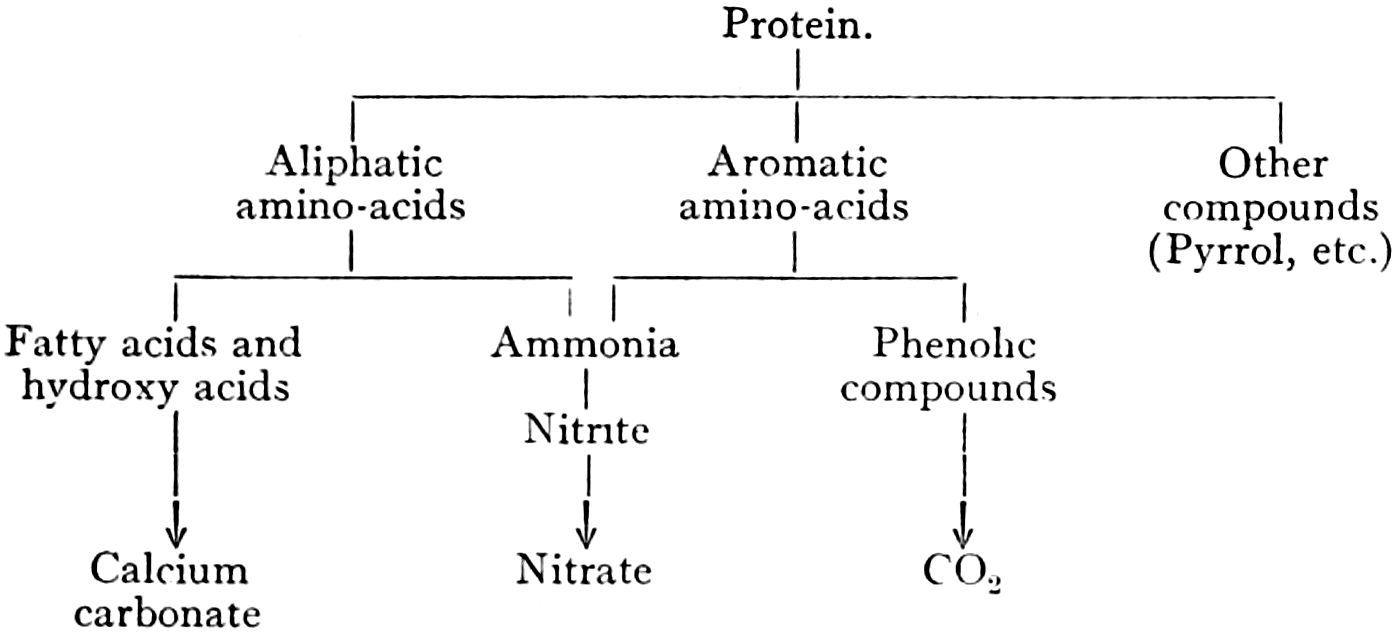
| Protein. | ||||||
| Aliphatic amino-acids |
Aromatic amino-acids |
Other compounds (Pyrrol, etc.) |
||||
| Fatty acids and hydroxy acids |
Ammonia | Phenolic compounds |
||||
| Nitrite | ||||||
| Calcium carbonate |
Nitrate | CO2 | ||||
It must be admitted that the evidence is indirect. The rate of oxidation of ammonia by bacteria in the soil is more rapid than the rate of formation, so that ammonia is practically never found in the soil in more than minimal amounts (1 or 2 parts per 1,000,000); indeed, the only evidence of its formation was for a long time the fact that no compound other than ammonia could be oxidised by the nitrifying organism. It has, however, since been shown at Rothamsted that ammonia accumulates in soils in which the nitrifying organism has been killed.
Nothing is known of the mechanism of the oxidation of ammonia beyond the fact that it is biological; the reaction is not easily effected chemically at ordinary temperatures. Possibly the organism assimilates ammonia at one end of a chain of metabolic processes and excretes nitrates at the other. Or, the reaction may be simply a straight oxidation for energy purposes, the ammonia changing to hydroxylamine and then to nitrous and nitric acids.
The nitrate does not remain long in the soil. Some is taken up by the plant and some is washed out from the soil. Part, however, either of the nitrate itself or of one of its precursors is converted into an insoluble form: probably it is changed into protein by the action of micro-organisms; it then goes through the whole process once more.
These are the general outlines; they present no particular chemical difficulties. When we come to details, however, there is much that cannot be understood.
[172]
First of all, there is the slow rate at which complex nitrogen compounds disappear from the soil in comparison with the rate of oxidation of the carbon. Thus, in the original plant residues, there is some forty times as much carbon as nitrogen: before they have been long in the soil there is only ten times as much carbon as nitrogen; this seems to be the stable position. What is the reason for this preferential oxidation of the carbon? No explanation can yet be given.

Fig. 24.
X-axis: 1887-8 1890-1 1900-1 1910-11
Y-axis: ℔ per acre
An equally difficult problem arises in connection with the length of time the process will continue. Decomposition of the nitrogen compounds never seems to be complete[173] in the soil; it dribbles on interminably. In the year 1870 Lawes and Gilbert cut off a block of soil from its surroundings and undermined it so that the drainage water could be collected and analysed. The soil has been kept free from vegetation or addition of nitrogen compounds from that time till now; yet it has never failed to yield nitrates, and the annual yield falls off only very slowly (Fig. 24). This same peculiarity is seen in the yield of crops on unmanured land: it decreases, but very gradually; even after eighty years the process is far from complete, and there is no sign that it will ever come to an end.
TABLE XVII.—APPROXIMATE LOSS OF NITROGEN FROM CULTIVATED SOILS: BROADBALK WHEAT FIELD, ROTHAMSTED, FORTY-NINE YEARS (1865-1914.)
| Rich Soil: Plot 2. Lb. per Acre. |
Poor Soil: Plot 3. Lb. per Acre. |
|||
|---|---|---|---|---|
| Nitrogen in soil in 1865 | ·175 per cent. = 4340 | ·105 per cent. = 2720 | ||
| Nitrogen added in manure, rain (5 lb. per annum), and seed (2 lb. per annum) | 10,140 | 340 | ||
| Nitrogen expected in 1914 | 14,480 | 3060 | ||
| Nitrogen found in 1914 | ·259 per cent. = 5950 | ·095 per cent. = 2590 | ||
| Loss from soil | 8530 | 470 | ||
| Nitrogen accounted for in crops | 2500 | 750 | ||
| Balance, being dead loss | 6030 | -280 | [J] | |
| Annual dead loss | 123 | - 6 | [J] | |
| [J] Gains. Possibly the result of bacterial action. | ||||
A further remarkable fact connected with the decomposition of the nitrogen compounds is that it seems invariably to be accompanied by an evolution of gaseous nitrogen. Apparently there are two cases. Under anaerobic conditions many of the soil organisms have the power of obtaining their necessary oxygen from nitrates, thereby causing a change in the molecule which leads in some cases to liberation of gaseous nitrogen; but the same result seems[174] to be attained in aerobic conditions, especially when carbon is being rapidly oxidised.
It is possible that the reaction is the same, and that in spite of the general aerobic conditions there is locally an anaerobic atmosphere. But it is also possible that some direct oxidation of protein or amino-acids may yield gaseous nitrogen. However it is brought about it affects a considerable proportion of the entire stock of nitrogen, and it becomes more serious as cultivation is intensified. Thus, on the Broadbalk plot receiving farmyard manure the loss is particularly heavy; on the unmanured plot it cannot be detected. The nitrogen balance-sheet is shown in Table XVII.
The oxidation of carbonaceous matter, however, is not invariably accompanied by a net loss of nitrogen; in other circumstances there is a net gain. In natural conditions there seems always to have been some leguminous vegetation growing; the gain may, therefore, be ascribed to the activity of the nodule organism. In pot experiments, however, it has been found possible, by adding sugar to the soil, to obtain gains of nitrogen where there is no leguminous vegetation, and this is attributed to the activity of Azotobacter.
The nitrogen cycle as observed in the soil is as follows:—
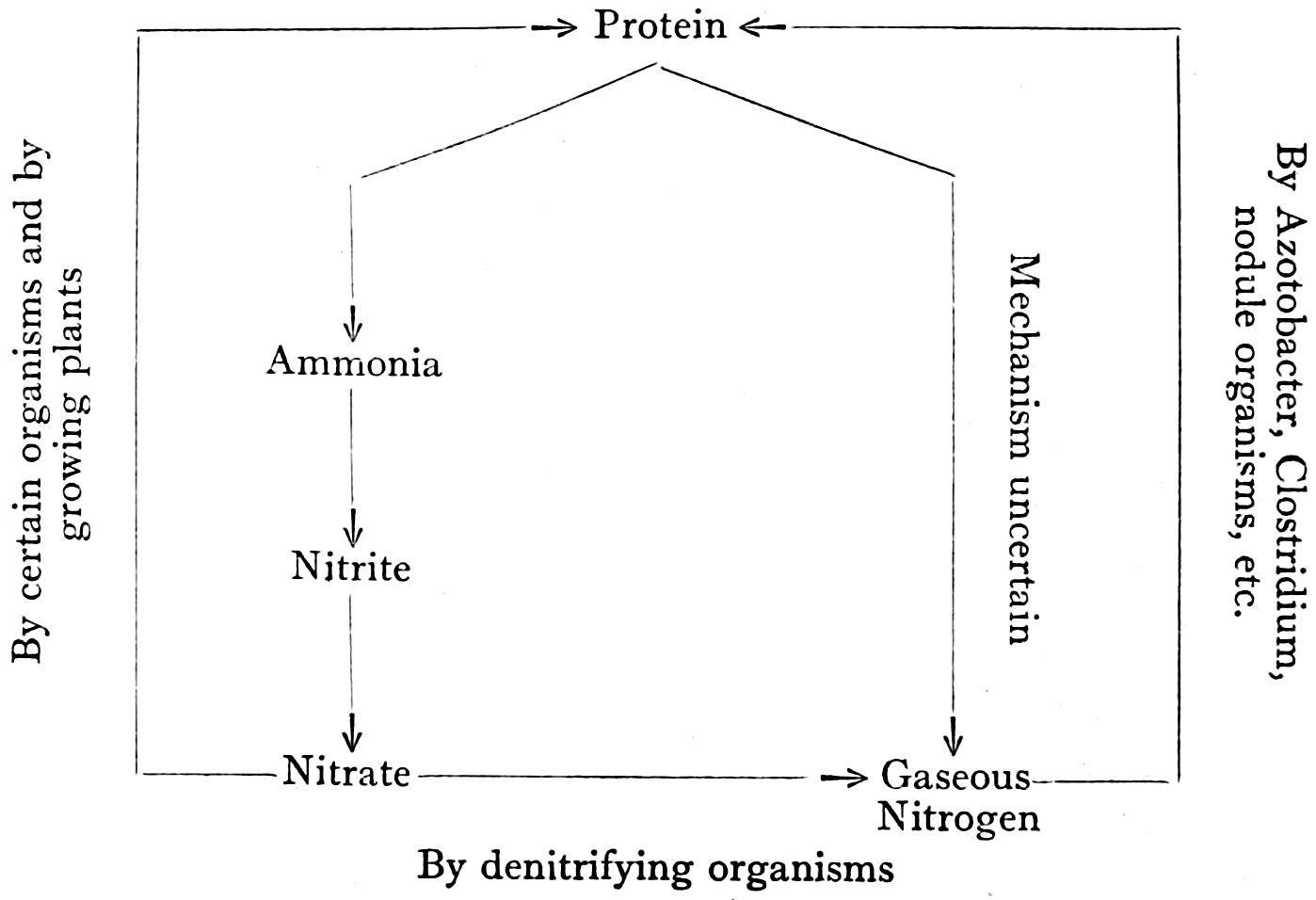
| Protein | |||
| By certain organisms and by growing plants | Ammonia | Mechanism uncertain | By Azotobacter, Clostridium, nodule organisms, etc. |
| Nitrite | |||
| Nitrate | Gaseous Nitrogen | ||
| By denitrifying organisms | |||
[175]
There has been but little study of the process of decomposition of the other compounds in plants. Part, if not all, of the sulphur is known to appear as sulphate, and some of the phosphorus as phosphate. It is certain that the plant constituents decompose, for there is no sign of their accumulation in the soil. They may exert transitory effects, but there is nothing to show permanent continuance. The toxic conditions which cause trouble in working with pure cultures of organisms in specific cultures media do not, so far as is known, arise in the soil. All attempts to find bacterio-toxins or plant toxins in normal soils have failed. The product toxic to one organism seems to be a useful nutrient to another, and so the mixed population keeps the soil healthy for all its members.
There is little precise knowledge as to the part played by the different members of the soil population in bringing about these changes.
We know in a general way that earthworms effect the distribution of the plant residues in the soil, and serve to disintegrate them; there is no evidence, however, that they play any indispensable part in the decomposition. Many root and other fragments do not go through this process; observation shows that fungi can force a way in, and they may be followed by nematodes which continue the disintegration. Possibly some of the flagellates help, and certainly the bacteria do. After that nothing is certain. We cannot, with certainty, assign any particular reaction in the decomposition to any specific organism, with the exception of the oxidation of the phenolic substances, the conversion of ammonia to nitrite and nitrate, and the fixation of nitrogen. With these exceptions many organisms seem capable of bringing about the reactions, and indeed some of the reactions may be purely chemical and independent of biological agencies.
The relationships between the soil population and soil fertility are readily stated in general outline, but they are by no means clear cut when one comes to details;[176] fertility is a complex property, and some of its factors are independent of soil micro-organisms.
The general relationship between plants and soil organisms is one of complete mutual interdependence. The growing plant fixes the sun’s energy and converts it into a form utilisable by the soil organisms; without the plant they could not exist. The plant is equally dependent on the soil organisms in at least two directions: their scavenging action removes the dead vegetation which would, if accumulated on the surface of the soil, effectively prevent most plants from growing. Further, the plant is dependent on the soil population for supplies of nitrates. Nothing is known about the relative efficiencies of the various soil organisms as scavengers. Numerous fungi and bacteria are effective producers of ammonia, the precursor of nitrates; it is not known, however, whether flagellates and such higher forms as nematodes act in this way.
This widespread power of producing ammonia makes it impossible in our present knowledge to regard any particular group of organisms as par excellence promoters of fertility. Indeed, it is safest not to attempt to do so. The primary purpose of the activities of a soil organism is to obtain energy and cell material for itself; any benefit to the plant is purely incidental. For cell material it must have nitrogen and phosphorus; here it competes with the plant. If it produces more ammonia than it utilises—in other words, if it is driven to nitrogen compounds for its energy, then the plant benefits. If, on the other hand, it absorbs more ammonia than it produces, as happens when it derives its energy from non-nitrogenous substances, the plant suffers. Thus, addition of peptone to the soil or an increase in bacterial numbers effected without addition of external energy (e.g. by partial sterilisation) leads to increased ammonia supply, and, therefore, to increased fertility. But addition of sugar to the soil causes so great an increase of numbers of bacteria and other organisms that considerable absorption[177] of ammonia and nitrate occurs, and fertility is for a time depressed.
Both actions proceed in soils partially sterilised by organic substances, such as phenol, which are utilised by some of the soil organisms; there is first a great rise in numbers of these particular organisms with a depression of ammonia and nitrate, then a drop to the new level, higher than the old one, and an increased production of ammonia and nitrate resulting from the partial sterilisation effects.
We must then regard the soil population as concerned entirely to maintain itself, and only incidently benefiting the plant, sometimes, indeed, injuring it; always essential, yet always taking its toll, and sometimes a heavy toll, of the plant nutrients it produces.
This effect makes it difficult to deduce simple quantitative relationships between bacterial activity and soil fertility, and the difficulty is increased by the fact that bacteria and plants may both be injured or benefited by the same causes, so that high bacterial numbers in a fertile soil would not necessarily be the cause, but might be simply the result of fertility.
The circumstance that certain soil organisms—bacteria, algæ, and fungi—themselves assimilate ammonia and nitrate may account for the remarkable slowness of nitrate accumulation, to which reference has already been made. The protein formed from the assimilated nitrogen remains in the bodies of the organisms, living or dead, till decomposition sets in. It is not difficult to picture a cycle of events in which much of the nitrate formed is at once reabsorbed by other organisms, and only little is actually thrown off into the soil. Such a process might continue almost interminably so long as any carbonaceous material remained.
Finally, we come to the very interesting problem—is it possible to control the population of the soil?
The problem may seem superfluous in view of the[178] difficulties just mentioned. Some aspects of it, however, are fairly clearly defined.
In the first instance, some organisms appear to be wholly harmful to the plant; among them are parasitic eelworms and fungi, and bacteria causing disease.
Control of these organisms can be brought about by partial sterilisation, and of all methods heat is the most effective, but it is costly, and attempts are now being made to replace it by chemical treatment. The results are promising, but the investigation is laborious; the organisms show specific relationships, and in finding a sufficiently potent and convenient poison it is necessary in each case to make an investigation into the relationship between chemical constitution and toxicity to the particular organism concerned. Formaldehyde is usually potent against fungi, and the cresols, and particularly their chlor- and chloronitro-derivatives, are potent against animals (eelworms, etc.).
One group of organisms is wholly beneficial, those associated with leguminous plants. Attempts have been made to increase their activities by inoculating the soil with more vigorous strains. The practical difficulties still remain very considerable, but there is hope that they may be overcome.
It is also possible to shift the balance of the soil population in certain directions. Special groups of soil organisms can be caused to multiply temporarily, if not permanently, by satisfying their particular requirements. Thus, when a soil has been heated above 100° C. it becomes specially suited to the growth of fungi, and quite unsuited to certain bacteria such as the nitrifying organisms and others; if this heated soil is infected with a normal soil population the fungi develop to a remarkable extent. The nodule organisms appear to be stimulated by addition of farmyard manure and of phosphates, and the phenol-destroying organisms by successive small additions of phenol.
Finally, quite apart from the control of disease organisms, it is possible to alter the soil population considerably by[179] partial sterilisation, using a temperature of only about 60° C., or a poison like toluene that favours few of the soil organisms. This problem has already been discussed in Chapter I.
The control of the soil population is still only in its infancy, but it already promises useful developments. It cannot, however, be too strongly insisted that the only sure basis of control is knowledge, and we cannot hope to push control further till we have learned much more about the soil population than we know at present.
[183]
PRINTED IN GREAT BRITAIN BY THE UNIVERSITY PRESS, ABERDEEN
Inconsistent and archaic or unusual spelling, capitalisation, italicisation, hyphenation, etc. have been retained, unless mentioned below. The names and classifications of the organisms as used in the book do not always conform to modern names and classifications; these have not been changed.
Depending on the hard- and software used and their settings, not all elements may display as intended.
Page 14, table, lower right hand cell: the data given add up to 8, not to 9.
Page 47, ·9 × ·18 in size: the source document does not include the units; presumably the sizes are in microns.
Page 118, endnote 8c (2×): this note does not exist.
Subject Index, entry Zygorrhynchus mœlleri: also refers to Zygorrhynchus vuilleminii.
Changes made:
Footnotes, tables and illustrations have been moved out of text paragraphs.
Several minor obvious typographical, punctuation and spelling errors (including accents) have been corrected silently. In several cases spelling differences (mainly of proper names) between the text and the index and endnotes have been standardised. In the indexes and in tables some ditto marks have been replaced with the dittoed text. Some page references have been corrected to indicate the correct page number.
Text in a dashed box is not present as such in the source document, but has been transcribed from the illustration for legibility and ease of reference. Some tables have been re-arranged or split to fit the available width.
Page 28, 29: MacBeth changed to McBeth as elsewhere (in the Author Index the entries MacBeth and McBeth have been merged).
Page 32, formula: 30 changed to 3O.
Page 58: From Barnfeild, ... changed to From Barnfield, ....
Page 85: closing bracket deleted after ... Table VII. and Fig. 13.
Page 90, Table VIII, column 5: 350·000 and 150·000 changed to 350,000 and 150,000.
Page 97: No creature lies or dies to itself, ... changed to No creature lives or dies to itself, ...
Page 104: Danske Aerofile Alghe changed to Danske Aërofile Alger.
Page 114: Recherche sulla Malattia del Riso ... changed to Ricerche sulla Malattia del Riso ....
Page 115: ... sur de polymorphisme ... changed to ... sur le polymorphisme ....
Page 116: literature notes 38 (Robbins) and 48 (Schindler) changed to 33 and 34 respectively.
Page 120: Zygorrhynchus vuillemini changed to Zygorrhynchus vuilleminii as elsewhere.
Page 126: references to Waksman[24] and [24e] changed to [25] and [25e].
Page 129: ... preparée de la pres de Russum ... changed to ... préparée de la terre humeuse du Spanderswoud, près de Bussum ....
Page 134: reference to Kohshi[24] changed to [34].
Page 143: Sämenbildung changed to Säurenbildung (entry 5); Wurzelbranderregern im Baden changed to Wurzelbranderregern im Boden (entry 11).
Page 144: ... Umwandlung von Aminosamen in Oxysämen ... changed to ... Umwandlung von Aminosäuren in Oxysäuren ....; ... Wirkungen der Schimmelze ... changed to ... Wirkungen der Schimmelpilze ....; Hydrogen-iron concentration changed to Hydrogen-ion concentration.
Page 145: Ztschr. f. Garungs. Physiol. changed to Ztschr. f. Gärungsphysiol.
Page 146: einige Pilze gegen Hemizellulosen changed to einiger Pilze gegen Hemicellulosen.
Page 157: Such responses are known chemotropism ... changed to Such responses are known as chemotropism ....
Page 170: ... alphatic amino-acids ... changed to ... aliphatic amino-acids ....