
The Project Gutenberg EBook of Scientific American Supplement, No. 467, December 13, 1884, by Various This eBook is for the use of anyone anywhere in the United States and most other parts of the world at no cost and with almost no restrictions whatsoever. You may copy it, give it away or re-use it under the terms of the Project Gutenberg License included with this eBook or online at www.gutenberg.org. If you are not located in the United States, you'll have to check the laws of the country where you are located before using this ebook. Title: Scientific American Supplement, No. 467, December 13, 1884 Author: Various Release Date: August 28, 2014 [EBook #46706] Language: English Character set encoding: ISO-8859-1 *** START OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN SUPP., DEC 13, 1884 *** Produced by Juliet and other, Juliet Sutherland, Wayne Hammond and the Online Distributed Proofreading Team at http://www.pgdp.net
7447
| Scientific American Supplement, Vol. XVIII No. 467. | NEW YORK, DECEMBER 13, 1884. | Scientific American Supplement, $5 a year. |
| Scientific American, established 1845. | Scientific American and Supplement, $7 a year. | |
The Berlin Academy of Industry and the Academy of Building were united in 1876 to form the Technical High School. It was found that the buildings were not sufficiently large for the great number of scholars, and arrangements were made for erecting new buildings affording better accommodations. The first design was made by Lucal, who, after his death, was succeeded by Hitzig, who died in 1821, and who was succeeded, in turn, by Mr. Raschdorff.
The main building is shown in the annexed cut, taken from the Illustrirte Zeitung. It is four stories high and 754 ft. long, and the middle and side wings are about 656 ft. deep, the portions between the wings being about 164 ft. deep. In the interior five square courts are arranged, of which two are at the right and two at the left, and are separated by intermediate building. The middle court in the central portion of the building is covered by a glass roof and forms a vestibule surrounded by arcades, the halls of which lead to different rooms. In the middle portion are the rooms for the officers, and the reading rooms. The courts are erected in brick with sgraffito ornamentation; and the front, sides, and rear are erected in sandstone on a granite base. The first story, or ground floor, is of a yellowish color, and the upper story is of a clear whitish-gray. The building is richly ornamented by statues, busts, reliefs, and groups representing the different architects, artists, scientists, etc.
The buildings of the University of Strassburg are arranged in two groups; one in the northern and the other in the southern part of the city. All the buildings of the medical department were erected in the neighborhood of the hospital, which is located between the south wall of the city and the River Ill.
In front of the old "Fischerthor," or Fishergate, the college house, or college building proper, in which are located the offices, lecture rooms, etc., was erected. A front perspective view of this building is shown in the lower part of the annexed cut, taken from the Illustrirte Zeitung. Behind 7448 this main building, and between the Universitäts and Goethe Strasse, the buildings of the Chemical Institute, the Physical Institute, with its tower; the Botanical Institute, with the gardens and hothouses, and the Astronomical Institute, with its observatory and movable dome, are located. These buildings were designed by the architects Hermann, Eggert, Brion, and Salomon, all of Strassburg.
The main building was designed by Prof. Warth, of Karlsruhe, and the style of the same is a noble Italian renaisance of the early period. Upon a base of red sandstone the basement is erected in freestone rustic masonry, upon which the first story is erected in smooth stone with conspicuous joints. The top story is constructed with arched windows separated by Ionic columns or pilasters. The central portion, which projects from the front of the building, has a grand staircase and two corner pavilions. The upper part of the central portion is constructed with fluted Corinthian columns, between which niches are provided, in which busts of the ideal representatives of the faculties are placed, viz., Homer, Paulus, Solon, Hippocrates, Aristotle, and Archimedes. Above the cornice, in the tympanum, is placed a group, of which Athene, with the torch of science, is the main figure. In the niches in the pavilions at the corners of the middle portion are the statues of Germania and Argentina, the representative of the free city of Strassburg. The pavilions at the ends of the building are ornamented by thirty-six statues of German scientists. The middle portion of the building directly beyond the grand staircase is occupied by a large open court, having a rich glass roof. The left part of the lower story is divided into lecture rooms, and the right side into rooms for the officers, etc. The collections are in the upper story, and the chapel, or main hall, is in the middle of the building.
The subject upon which I am to speak to you this evening is happily for me not new in Philadelphia. The beautiful lectures on light which were given several years ago by President Morton, of the Stevens Institute, and the succession of lectures on the same subject so admirably illustrated by Prof. Tyndall, which many now present have heard, have fully prepared you for anything I can tell you this evening in respect to the wave theory of light.
It is indeed my humble part to bring before you some mathematical and dynamical details of this great theory. I cannot have the pleasure of illustrating them to you by anything comparable with the splendid and instructive experiments which many of you have already seen. It is satisfactory to me to know that so many of you now present are so thoroughly prepared to understand anything I can say, that those who have seen the experiments will not feel their absence at this time. At the same time I wish to make them intelligible to those who have not had the advantages to be gained by a systematic course of lectures. I must say in the first place, without further preface, as time is short and the subject is long, simply that sound and light are both due to vibrations propagated in the manner of waves; and I shall endeavor in the first place to define the manner of propagation and mode of motion that constitute those two subjects of our senses, the sense of sound and the sense of light.
Each is due to vibrations. The vibrations of light differ widely from the vibrations of sound. Something that I can tell you more easily than anything in the way of dynamics or mathematics respecting the two classes of vibrations is, that there is a great difference in the frequency of the vibrations of light when compared with the frequency of the vibrations of sound. The term "frequency," applied to vibrations, is a convenient term, applied by Lord Rayleigh in his book on sound to a definite number of full vibrations of a vibrating body per unit of time. Consider, then, in respect to sound, the frequency of the vibrations of notes, which you all know in music represented by letters, and by the syllables for singing the do, re, mi, etc. The notes of the modern scale correspond to different frequencies of vibrations. A certain note and the octave above it correspond to a certain number of vibrations per second and double that number.
I may explain in the first place conveniently the note called "C;" I mean the middle "C." I believe it is the C of the tenor voice, that most nearly approaches the tones used in speaking. That note corresponds to two hundred and fifty-six full vibrations per second, two hundred and fifty-six times to and fro per second of time.
Think of one vibration per second of time. The seconds pendulum of the clock performs one vibration in two seconds, or a half vibration in one direction per second. Take a 10-inch pendulum of a drawing-room clock, which vibrates twice as fast as the pendulum of an ordinary eight-day clock, and it gives a vibration of one per second, a full period of one per second to and fro. Now think of three vibrations per second. I can move my hand three times per second easily, and by a violent effort I can move it to and fro five times per second. With four times as great force, if I could apply it, I could move it twice five times per second.
Let us think, then, of an exceedingly muscular arm that would cause it to vibrate ten times per second, that is, ten times to the left and ten times to the right. Think of twice ten times, that is, twenty times per second, which would require four times as much force; three times ten, or thirty times a second, which require nine times as much force. If a person were nine times as strong as the most muscular arm can be, he could vibrate his hand to and fro thirty times per second, and without any other musical instrument could make a musical note by the movement of his hand which would correspond to one of the pedal notes of an organ.
If you want to know the length of a pedal pipe, you can calculate it in this way. There are some numbers you must remember, and one of them is this. You, in this country, are subjected to the British insularity in weights and measures; you use the foot and inch and yard. I am obliged to use that system, but I apologize to you for doing so, because it is so inconvenient, and I hope all Americans will do everything in their power to introduce the French metrical system. I hope the evil action performed by an English minister whose name I need not mention, because I do not wish to throw obloquy on any one, may be remedied. He abrogated a useful rule, which for a short time was followed and which I hope will soon be again enjoined, that the French metrical system be taught in all our national schools. I do not know how it is in America. The school system seems to be very admirable, and I hope the teaching of the metrical system will not be let slip in the American schools any more than the use of the globes.
I say this seriously. I do not think any one knows how seriously I speak of it. I look upon our English system as a wickedly brain-destroying piece of bondage under which we suffer. The reason why we continue to use it is the imaginary difficulty of making a change, and nothing else; but I do not think that in America any such difficulty should stand in the way of adopting so splendidly useful a reform.
I know the velocity of sound in feet per second. If I remember rightly, it is 1,089 feet per second in dry air at the freezing point, and 1,115 feet per second in air of what we call moderate temperature, 59 or 60 degrees (I do not know whether that temperature is ever attained in Philadelphia or not; I have had no experience of it, but people tell me it is sometimes 59 or 60 degrees in Philadelphia, and I believe them); in round numbers let us call it 1,000 feet per second. Sometimes we call it a thousand musical feet per second, it saves trouble in calculating the length of organ pipes; the time of vibration in an organ pipe is the time it takes a vibration to run from one end to the other and back. In an organ pipe 500 feet long the period would be one per second; in an organ pipe 10 feet long the period would be 50 per second; in an organ pipe 20 feet long the period would be 25 per second at the same rate. Thus 25 per second and 50 per second of frequencies correspond to the periods of organ pipes of 20 feet and 10 feet.
The period of vibration of an organ pipe, open at both ends, is approximately the time it takes sound to travel from one end to the other and back. You remember that the velocity in dry air in a pipe 10 feet long is a little more than 50 periods per second; going up to 256 periods per second, the vibrations correspond to those of a pipe 2 feet long. Let us take 512 periods per second; that corresponds to a pipe about a foot long. In a flute, open at both ends, the holes are so arranged that the length of the sound wave is about 1 foot, for one of the chief "open notes." Higher musical notes correspond to greater and greater frequency of vibration, viz., 1,000, 2,000, 4,000 vibrations per second; 4,000 vibrations per second correspond to a piccolo flute of exceedingly small length; it would be but one and a half inches long. Think of a note from a little dog call, or other whistle one and a half inches long, open at both ends, or from a little key having a tube three-quarters of an inch long, closed at one end; you will then have 4,000 vibrations per second.
A wave length of sound is the distance traversed in the period of vibration. I will illustrate what the vibrations of sound are by this condensation traveling along our picture on the screen. Alternate condensations and rarefactions of the air are made continuously by a sounding body. When I pass my hand vigorously in one direction, the air before it becomes dense, and the air on the other side becomes rarefied. When I move it in the other direction, these things become reversed; there is a spreading out of condensation from the place where my hand moves in one direction and then in the reverse. Each condensation is succeeded by a rarefaction. Rarefaction succeeds condensation at an interval of one-half what we call "wave lengths." Condensation succeeds condensation at the full interval of what we call wave lengths.
We have here these luminous particles on this scale,2 representing portions of the air close together, dense; a little higher up, portions of air less dense. I now slowly turn the handle of the apparatus in the lantern, and you see the luminous sectors showing condensation traveling slowly upward on the screen; now you have another condensation; making one wave length.
This picture or chart represents a wave length of four feet. It represents a wave of sound four feet long. The fourth part of a thousand is 250. What we see now of the actual scale represents the lower note C of the tenor voice. The air from the mouth of a singer is alternately condensed and rarefied just as you see here.
But that process shoots forward at the rate of one thousand feet per second; the exact period of the motion is 256 vibrations per second for the actual case before you. Follow one particle of the air forming part of a sound wave, as represented by these moving spots of light on the screen; now it goes down, then another portion goes down rapidly; now it stops going down; now it begins to go up; now it goes down and up again.
As the maximum of condensation is approached, it is going up with diminishing maximum velocity. The maximum of rarefaction has now reached it, and the particle stops going up and begins to move down. When it is of mean density the particles are moving with maximum velocity, one way or the other. You can easily follow these motions, and you will see that each particle moves to and fro, and the thing that we call condensation travels along.
I shall show the distinction between these vibrations and the vibrations of light. Here is the fixed appearance of the particles when displaced but not in motion. You can imagine particles of something, the thing whose motion constitutes light. This thing we call the luminiferous ether. That is the only substance we are confident of in dynamics. One thing we are sure of, and that is the reality and substantiality of the luminiferous ether. This instrument is merely a method of giving motion to a diagram designed for the purpose of illustrating wave motion of light. I will show you the same thing in a fixed diagram, but this arrangement shows the mode of motion.
Now follow the motion of each particle. This represents a particle of the luminiferous ether, moving at the greatest speed when it is at the middle position.
You see two modes of vibration,3 sound and light now moving together—the traveling of the wave of condensation and rarefaction, and the traveling of the wave of transverse displacement. Note the direction of propagation. Here it is from your left to your right, as you look at it. Look at the motion when made faster. We have now the direction reversed. The propagation of the wave is from right to left, again the propagation of the wave is from left to right; each particle moves perpendicularly to the line of propagation.
I have given you an illustration of the vibration of sound waves, but I must tell you that the movement illustrating the condensation and rarefaction represented in that moving diagram are necessarily very much exaggerated to let the motion be perceptible, whereas the greatest condensation in actual sound motion is not more than one or two per cent, or a small fraction of a per cent. Except that the amount of condensation was exaggerated in the diagram for sound, you have a correct representation of what actually takes in the low note C.
On the other hand, in the moving diagram representing light waves what had we? We had a great exaggeration of the inclination of the line of particles. You must first imagine a line of particles in a straight line, and then you must imagine them disturbed into a wave curve, the shape of the curve corresponding to the disturbance. Having seen what the propagation of the wave is, look at this diagram and then look at that one. This, in light, corresponds to the different sounds I spoke of at first. The wave length of light is the distance from crest to crest of the wave, or from hollow to hollow. I speak of crests and hollows, because we have a diagram of ups and downs as the diagram is placed.
Here, then, you have a wave length.4 In this lower diagram you have the wave length of violet light. It is but one-half the length of the upper wave of red light; the period of vibration is but half as long. Now, on an enormous scale, exaggerated not only as to slope, but immensely magnified as to wave length, we have an illustration of the waves of light. The drawing marked "red" corresponds to red light, and this lower diagram corresponds to violet light. The upper curve really corresponds to something a little below the red ray of light in the spectrum, and the lower curve to something beyond the violet light. The variation in length between the most extreme rays is in the proportion of four and a half of red to eight of the violet, instead of four and eight; the red waves are nearly as one to two of the violet.
To make a comparison between the number of vibrations for each wave of sound and the number of vibrations constituting light waves, I may say that 30 vibrations per second is about the smallest number which will produce a musical sound; 50 per second give one of the grave pedal notes of an organ, 100 or 200 per second give the low notes of the bass voice, higher notes with 250 per second, 300 per second, 1,000, 4,000, up to 8,000 per second, give about the shrillest notes audible to the human ear.
Instead of the numbers, which we have, say, in the most commonly used part of the musical scale, i. e., from 200 or 300 to 600 or 700 per second, we have millions and millions of vibrations per second in light waves; that is to say, 400 million million per second, instead of 400 per second. That number of vibrations is performed when we have red light produced.
An exhibition of red light traveling through space from the remotest star is due to the propagation by waves or vibrations, in which each individual particle of the transmitting medium vibrates to and fro 400 million million times in a second.
Some people say they cannot understand a million million. Those people cannot understand that twice two makes four. That is the way I put it to people who talk to me about the incomprehensibility of such large numbers. I say finitude is incomprehensible, the infinite in the universe is comprehensible. Now apply a little logic to this. Is the negation of infinitude incomprehensible? What would you think of a universe in which you could travel one, ten, or a thousand miles, or even to California, and then find it come to an end? Can you suppose an end of matter, or an end of space? The idea is incomprehensible. Even if you were to go millions and millions of miles, the idea of coming to an end is incomprehensible.
You can understand one thousand per second as easily as you can understand one per second. You can go from one to ten, and ten times ten and then to a thousand without taxing your understanding, and then you can go on to a thousand million and a million million. You can all understand it.
Now 400 million million vibrations per second is the kind of thing that exists as a factor in the illumination by red light. Violet light, after what we have seen and have illustrated by that curve, I need not tell you corresponds to vibrations of 800 million million per second. There are recognizable qualities of light caused by vibrations of much greater frequency and much less frequency than this. You may imagine vibrations having about twice the frequency of violet light and one fifteenth the frequency of red light, and still you do not pass the limit of the range of continuous phenomena only a part of which constitutes visible light.
Everybody knows the "photographer's light," and has heard of invisible light producing visible effects upon the chemically prepared plate in the camera. Speaking in round numbers, I may say that, in going up to about twice the frequency I have mentioned for violet light, you have gone to the extreme end of the range of known light of the highest rates of vibration; I mean to say that you have reached the greatest frequency that has yet been observed.
When you go below visible red light, what have you? We have something we do not see with the eye, something that the ordinary photographer does not bring out on his photographically sensitive plates. It is light, but we do not see it. It is something so closely continuous with light visible, that we may define it by the name of invisible light. It is commonly called radiant heat; invisible radiant heat. Perhaps, in this thorny path of logic, with hard words flying in our faces, the least troublesome way of speaking of it is to call it radiant heat. The heat effect you experience when you go near a bright, hot coal fire, or a hot steam boiler; or when you go near, but not over, a set of hot water pipes used for heating a house; the thing we perceive in our face and hands when we go near a boiling pot and hold the hand on a level with it, is radiant heat; the heat of the hands and face caused by a hot fire, or a hot kettle when held under the kettle, is also radiant heat.
You might readily make the experiment with an earthen teapot; it radiates heat better than polished silver. Hold your hands below, and you perceive a sense of heat; above the teapot you get more heat; either way you perceive heat. If held over the teapot, you readily understand that there is a little current of air rising. If you put your hand under the teapot, you get cold air; the upper side of your hand is heated by radiation, while the lower side is fanned and is actually cooled by virtue of the heated kettle above it. 7449
That perception by the sense of heat is the perception of something actually continuous with light. We have knowledge of rays of radiant heat perceptible down to (in round numbers) about four times the wave length, or one-fourth the period of visible or red light. Let us take red light at 400 million million vibrations per second; then the lowest radiant heat, as yet investigated, is about 100 million million per second in the way of frequency of vibration.
I had hoped to be able to give you a lower figure. Prof. Langley has made splendid experiments on the top of Mount Whitney, at the height of 1,500 feet above the sea level, with his "bolometer," and has made actual measurements of the wave lengths of radiant heat down to exceedingly low figures. I will read you one of the figures; I have not got it by heart yet, because I am expecting more from him.5 I learned a year and a half ago that the lowest radiant heat observed by the diffraction method of Prof. Langley corresponded to 28 one-hundred-thousandths of a centimeter for wave length, 28 as compared with red light, which is 7.3, or nearly fourfold. Thus wave lengths of four times the amplitude or one-fourth the frequency per second of red light have been experimented on by Prof. Langley, and recognized as radiant heat.
Photographic or actinic light, as far as our knowledge extends at present, takes us to a little less than one-half the wave length of violet light. You will thus see that while our acquaintance with wave motion below the red extends down to one-quarter of the slowest rate which affects the eye, our knowledge of vibrations at the other end of the scale only comprehends those having twice the frequency of violet light. In round numbers, we have four octaves of light, corresponding to four octaves of sound in music. In music the octave has a range to a note of double frequency. In light we have one octave of visible light, one octave above the visible range, and two octaves below the visible range. We have one hundred per second, two hundred per second, four hundred per second (million million understood) for invisible radiant heat, eight hundred per second for visible light, and one thousand six hundred per second for invisible light.
One thing in common to the whole is the heat effect. It is extremely small in moonlight, so small that nobody until recently knew there was any heat in the moon's rays. Herschel thought it was perceptible in our atmosphere by noticing that it dissolved away very light clouds, an effect which seemed to show in full moonlight more than when we have less than full moon. Herschel, however, pointed this out as doubtful, but now, instead of its being a doubtful question, we have Prof. Langley giving as a fact that the light from the moon drives the indicator of his sensitive instrument clear across the scale, and with a comparatively prodigious heating effect!
I must tell you that if any of you want to experiment with the heat of the moonlight, you must compare the heat with whatever comes within the influence of the moon's rays only. This is a very necessary precaution; if, for instance, you should take your bolometer or other heat detecter from a comparatively warm room into the night air, you would obtain an indication of a fall in temperature owing to this change. You must be sure that your apparatus is in thermal equilibrium with the surrounding air, then take your burning glass, and first point it to the moon and then to space in the sky beside the moon; you thus get a differential measurement in which you compare the radiation of the moon with the radiation of the sky. You will then see that the moon has a distinctly heating effect.
To continue our study of visible light, that is, undulations extending from red to violet in the spectrum (which I am going to show you presently), I would first point out on this chart that in the section from letter A to letter D, we have visual effect and heating effect only; but no ordinary chemical or photographic effect.
Photographers can leave their usual sensitive, chemically prepared plates exposed to yellow light and red light without experiencing any sensible effect; but when you get toward the blue end of the spectrum, the photographic effect begins to tell, more and more as you get toward the violet end. When you get beyond the violet, there is the invisible light known chiefly by its chemical action. From yellow to violet we have visual effect, heating effect, and chemical effect, all three; above the violet, only chemical and heating effect, and so little of the heating effect that it is scarcely perceptible.
The prismatic spectrum is Newton's discovery of the composition of white light. White light consists of every variety of color from red to violet. Here, now, we have Newton's prismatic spectrum produced by a prism. I will illustrate a little in regard to the nature of color by putting something before the light which is like colored glass; it is colored gelatin. I will put in a plate of red gelatin which is carefully prepared of chemical materials, and see what that will do. Of all the light passing to it from violet to red, it only lets through the red and orange, giving a mixed reddish color.
Here is another plate of green gelatin. The green absorbs all the red, giving only green. Here is another plate absorbing something from each portion of the spectrum, taking away a great deal of the violet and giving a yellow or orange appearance to the light. Here is another absorbing out the green, leaving red, orange, and a very little faint green, and absorbing out all the violet.
When the spectrum is very carefully produced, far more perfectly than Newton knew how to show it, we have a homogeneous spectrum. It must be noticed that Newton did not understand what we call a homogeneous spectrum; he did not produce it, and does not point out in his writings the conditions for producing it. With an exceedingly fine line of light we can bring it out as in sunlight, like this upper picture, red, orange, yellow, green, blue, indigo, and violet according to Newton's nomenclature. Newton never used a narrow beam of light, and so could not have had a homogeneous spectrum.
This is a diagram painted on glass and showing the colors as we know them. It would take two or three hours if I were to explain the subject of spectrum analysis to-night. We must tear ourselves away from it. I will just read out to you the wave lengths corresponding to the different positions in the sun's spectrum of certain dark lines commonly called "Fraunhofer's lines." I will take as a unit the one-hundred-thousandth of a centimeter. A centimeter is 0.4 of an inch; it is a rather small half an inch. I take the thousandth of a centimeter and the hundred of that as a unit. At the red end of the spectrum the light in the neighborhood of that black line A has for its wave length 7.6; B has 6.87; D has 5.89; the "frequency" for A is 3.9 times 100 million million; the frequency of D light is 5.1 times 100 million million per second.
Now, what force is concerned in those vibrations as compared with sound at the rate of 400 vibrations per second? Suppose for a moment the same matter was to move to and fro through the same range but 400 million million times per second. The force required is as the square of the number expressing the frequency. Double frequency would require quadruple force for the vibration of the same body. Suppose I vibrate my hand again, as I did before. If I move it once per second, a moderate force is required; for it to vibrate ten times per second, 100 times as much force is required; for 400 vibrations per second, 160,000 times as much force.
If I move my hand once per second through a space of a quarter of an inch; a very small force is required; it would require very considerable force to move it ten times a second, even through so small a range; but think of the force required to move a tuning fork 400 times a second; compare that with the force required for a motion of 400 million million times a second. If the mass moved is the same, and the range of motion is the same, then the force would be one million million million million times as great as the force required to move the prongs of the tuning fork. It is as easy to understand that number as any number like 2, 3, or 4.
Consider gravely what that number means, and what we are to infer from it. What force is there in space between my eye and that light? What forces are there in space between our eyes and the sun and our eyes and the remotest visible star! There is matter and there is motion, but what magnitude of force may there be?
I move through this "luminiferous ether" as if it were nothing. But were there vibrations with such frequency in a medium of steel or brass, they would be measured by millions and millions and millions of tons action on a square inch of matter. There are no such forces in our air. Comets make a disturbance in the air, and perhaps the luminiferous ether is split up by the motion of a comet through it. So when we explain the nature of electricity, we explain it by a motion of the luminiferous ether. We cannot say that it is electricity. What can this luminiferous ether be? It is something that the planets move through with the greatest ease. It permeates our air; it is nearly in the same condition, so far as our means of judging are concerned, in our air and in the interplanetary space. The air disturbs it but little; you may reduce the air by air pumps to the hundred thousandth of its density, and you make little effect in the transmission of light through it. The luminiferous ether is an elastic solid. The nearest analogy I can give you is this jelly which you see.6 The nearest analogy to the waves of light is the motion, which you can imagine, of this elastic jelly, with a ball of wood floating in the middle of it. Look there, when with my hand I vibrate the little red ball up and down, or when I turn it quickly round the vertical diameter, alternately in opposite directions; that is the nearest representation I can give you of the vibrations of luminiferous ether.
Another illustration is Scottish shoemaker's wax or Burgundy pitch, but I know Scottish shoemaker's wax better. It is heavier than water, and absolutely answers my purpose. I take a large slab of the wax, place it in a glass jar filled with water, place a number of corks on the lower side and bullets on the upper side. It is brittle like the Trinidad or Burgundy pitch which I have in my hand. You can see how hard it is, but if left to itself it flows like a fluid. The shoemaker's wax breaks with a brittle fracture, but it is viscous, and gradually yields.
What we know of the luminiferous ether is that it has the rigidity of a solid, and gradually yields. Whether or not it is brittle and cracks we cannot yet tell, but I believe the discoveries in electricity, and the motions of comets and the marvelous spurts of light from them, tend to show cracks in the luminiferous ether—show a correspondence between the electric flash and the aurora borealis and cracks in the luminiferous ether. Do not take this as an assertion, it is hardly more than a vague scientific dream; but you may regard the existence of the luminiferous ether as a reality of science, that is, we have an all-pervading medium, an elastic solid, with a great degree of rigidity; its rigidity is so prodigious in proportion to its density that the vibrations of light in it have the frequencies I have mentioned, with the wave lengths I have mentioned.
The fundamental question as to whether or not luminiferous ether has gravity has not been answered. We have no knowledge that the luminiferous ether is attracted by gravity; it is sometimes called imponderable because some people vainly imagine that it has no weight. I call it matter with the same kind of rigidity that this elastic jelly has.
Here are two tourmalines; if you look through them toward the light, you see the white light all around, i. e., they are transparent. If I turn round one of these tourmalines the light is extinguished, it is absolutely black, as though the tourmalines were opaque. This is an illustration of what is called polarization of light. I cannot speak to you about qualities of light without speaking of the polarization of light. I want to show you a most beautiful effect of polarizing light, before illustrating a little further by means of this large mechanical illustration which you have in the bowl of jelly. Now I put in the lantern another instrument called a "Nicol prism." What you saw first were two plates of the crystal tourmaline which came from Brazil, I believe, having the property of letting light pass when both plates are placed in one particular direction as regards their axes of crystallization, and extinguishing it when it passes through the first plate held in another direction. We have now an instrument which also gives rays of polarized light. A Nico prism is a piece of Iceland spar, cut in two and turned, one part relatively to the other, in a very ingenious way, and put together again, and cemented into one by Canada balsam. The Nicol prism takes advantage of the property which the spar has of double refraction, and produces the phenomenon which I now show you.
I turn one prism round in a certain direction and you get light, a maximum of light. I turn it through a right angle and you get blackness. I turn it one-quarter round again and get maximum light; one-quarter more, maximum blackness; one-quarter more, and bright light. We rarely have such a grand specimen of a Nicol prism as this.
There is another way of producing polarized light. I stand before that light, and look at its reflection in a plate of glass on the table through one of the Nicol prisms, which I turn round, so. Now I must incline that piece of glass at a particular angle, rather more than forty-five degrees; I find a particular angle in which, if I look at it and then turn the prism round in the hand, the effect is absolutely to extinguish the light in one position and to give it maximum brightness in another position. I use the term "absolute" somewhat rashly. It is only a reduction to a very small quantity of light, not an absolute annulment as we have in the case of the two Nicol prisms used conjointly. Those of you who have never heard of this before would not know what I am talking about. As to the mechanics of the thing, it could only be explained to you by a course of lectures on physical optics. The thing is this: vibrations of light must be in a definite direction relatively to the line in which the light travels.
Look at this diagram: the light goes from left to right; we have vibrations perpendicular to the line of transmission. There is a line up and down, which is the line of vibration. Imagine here a source of light, violet light, and here in front of it is the line of propagation. Sound vibrations are to and fro; this is transverse to the line of propagation. Here is another, perpendicular to the diagram, still following the law of transverse vibration; here is another circular vibration. Imagine a long rope: you whirl one end of it, and you send a screw-like motion running along; you can get the circular motion in one direction or in the opposite.
Plane polarized light is light with the vibrations all in a single plane, perpendicular to the plane through the ray, which is technically called the "plane of polarization." Circular polarized light consists of undulations of luminiferous ether having a circular motion. Elliptically polarized light is something between the two, not in a straight line, and not in a circular line; the course of vibration is an ellipse. Polarized light is light that performs its motions continually in one mode or direction. If in a straight line, it is plane polarized; if in a circular direction, it is circularly polarized light; when elliptical, it is elliptically polarized light.
With Iceland spar, one unpolarized ray of light divides on entering it into two rays of polarized light, by reason of its power of double refraction, and the vibrations are perpendicular to one another in the two emerging rays. Light is always polarized when it is reflected from a plate of unsilvered glass, or water, at a certain definite angle of fifty-six degrees for glass, fifty-two degrees for water, the angle being reckoned in each case from a perpendicular to the surface. The angle for water is the angle whose tangent is 1.4. I wish you to look at the polarization with your own eyes. Light from glass at fifty six degrees and from water at fifty-two degrees goes away vibrating perpendicularly to the plane of incidence and plane of reflection.
We can distinguish it without the aid of an instrument. There is a phenomenon well known in physical optics as "Haidinger's brushes." The discoverer is well known in Philadelphia as a mineralogist, and the phenomenon I speak of goes by his name. Look at the sky in a direction of ninety degrees from the sun, and you will see a yellow and blue cross, with the yellow toward the sun, and from the sun, spreading out like two foxes' tails with blue between, and then two red brushes in the space at right angles to the blue. If you do not see it, it is because your eyes are not sensitive enough, but a little training will give them the needed sensitiveness.
If you cannot see it in this way, try another method. Look into a pail of water with a black bottom; or take a clear glass dish of water, rest it on a black cloth and look down at the surface of the water on a day with a white cloudy sky (if there is such a thing ever to be seen in Philadelphia). You will see the white sky reflected in the basin of water at an angle of about fifty degrees. Look at it with the head tipped to one side, and then again with the head tipped to the other side, keeping your eyes on the water, and you will see Haidinger's brushes. Do not do it fast, or you will make yourself giddy. The explanation of this is the refreshing of the sensibility of the retina. The Haidinger's brush is always there, but you do not see it because your eye is not sensitive enough. After once seeing it, you always see it; it does not thrust itself inconveniently before you when you do not want to see it. You can readily see it in a piece of glass with dark cloth below it, or in a basin of water.
I am going to conclude by telling you how we know the wave lengths of light and how we know the frequency of the vibrations. We shall actually make a measurement of the wave length of the yellow light. I am going to show you the diffraction spectrum.
You see on the screen,7 on each side of a central white bar of light, a set of bars of light variegated colors, the first one, on each side, showing blue or indigo color, about four inches from the central white bar and red about four inches farther, with vivid green between the blue and the red. That effect is produced by a grating with 400 lines to the centimeter, engraved on glass, which I now hold in my hand. The next grating has 3,000 lines on a Paris inch. You see the central space, and on each side a large number of spectrums, blue at one end and red at the other. The fact that, in the first spectrum, red is about twice as far from the center as the blue, proves that a wave length of red light is double that of blue light.
I will now show you the operation of measuring the length of a wave of sodium light, that is, a light like that marked D on the spectrum, a light produced by a spirit lamp with salt in it. The sodium vapor is heated up to several thousand degrees, when it becomes self-luminous, and gives such a light as we get by throwing salt upon a spirit lamp in the game of snap dragon.
I hold in my hand a beautiful grating of glass silvered by Liebig's process of metallic silver, a grating with 6,480 lines to the inch, belonging to my friend Prof. Barker, which he has kindly brought here for us this evening. You will see the brilliancy of color as I turn the light reflected from the grating toward you and pass the beam around the room. You have now seen directly with your own eyes these brilliant colors reflected from the grating, and you have also seen them thrown upon the screen from a grating placed in the lantern. With a grating of 17,000 lines, a much greater number of lines per inch than the other, you will see how much further from the central bright space the first spectrum is; how much more this grating changes the direction or diffraction of the beam of light. Here is the center of the grating, and there is the first spectrum. You will note that the violet light is least diffracted and the red light is most 7450 diffracted. This diffraction of light first proved to us definitely the reality of the undulatory theory of light.
You ask, Why does not light go round the corner as sound does? Light goes round a corner in these diffraction spectrums; it is shown going round a corner, it passes through these bars and is turned round an angle of thirty degrees. Light going round a corner by instruments adapted to show the result, and to measure the angles at which it is turned, is called the diffraction of light.
I can show you an instrument which will measure the wave lengths of light. Without proving the formula, let me tell it to you. A spirit lamp with salt sprinkled on the wick gives very nearly homogeneous light, that is to say, light all of one wave length, or all of the same period. I have a little grating that I take in my hand. I look through this grating, and see that candle before me. Close behind it you see a blackened slip of wood with two white marks on it ten inches asunder. The line on which they are marked is placed perpendicular to the line at which I shall go from it. When I look at this salted spirit lamp, I see a series of spectrums of yellow light. As I am somewhat short-sighted, I am making my eye see with this eye-glass and the natural lenses of the eye what a long-sighted person would make out without an eye-glass. On that screen you saw a succession of spectrums. I now look direct at the candle, and what do I see? I see a succession of five or six brilliantly colored spectrums on each side of the candle. But when I look at the salted spirit lamp, now I see ten spectrums on one side and ten on the other, each of which is a monochromatic band of light.
I will measure the wave lengths of light thus: I walk away to a considerable distance, and look at the candle and marks. I see a set of spectrums. The first white line is exactly behind the candle. I want the first spectrum to the right of that white line to fall exactly on the other white line, which is ten inches from the first. As I walk away from it, I see it is now very near it; it is now on it. Now the distance from my eye is to be measured, and the problem is again to reduce feet to inches. The distance from the spectrum of the flame to my eye is thirty-four feet nine inches. Mr. President, how many inches is that? Four hundred and seventeen inches, in round numbers 420 inches. Then we have the proportion, as 420 is to 10 so is the length from bar to bar of the grating to the wave length of sodium light—that is to say, as forty-two is to one. The distance from bar to bar is the four-hundredth of a centimeter; therefore the 42d part of the four-hundredth of a centimeter is the required wave length, or the 16,800th of a centimeter is the wave length according to our simple, and easy, and hasty experiment. The true wave length of sodium light, according to the most accurate measurement, is about a 17,000th of a centimeter, which differs by scarcely more than one per cent. from our result!
The only apparatus you see is this little grating; it is a piece of glass with four-tenths of an inch ruled with 400 fine lines. Any of you who will take the trouble to buy one may measure the wave lengths of a candle flame himself. I hope some of you will be induced to make the experiment for yourselves.
If I put salt on the flame of a spirit lamp, what do I see through this grating? I see merely a sharply defined yellow light, constituting the spectrum of vaporized sodium, while from the candle flame I see an exquisitely colored spectrum, far more beautiful than I showed you on the screen. I see, in fact, a series of spectrums on the two sides with the blue toward the candle flame and the red further out. I cannot get one definite thing to measure from in the spectrum from the candle flame as I can with the flame of a spirit lamp with the salt thrown on it, which gives, as I have said, a simple yellow light. The highest blue light I see in the candle flame is now exactly on the line. Now measure to my eye; it is forty-four feet four inches, or 532 inches. The length of this wave then is the 532d part of the four-hundredth of a centimeter, which would be the 21,280th of a centimeter, say the 21,000th of a centimeter. Then measure for the red, and you would find something like the 11,000th for the lowest of the red light.
Lastly, how do we know the frequency of vibration?
Why, by the velocity of light. How do we know that? We know it in a number of different ways, which I cannot explain now because time forbids. Take the velocity of light. It is 187,000 British statute miles per second. But it is much better to take a kilometer for the unit. That is about six-tenths of a mile. The velocity is very accurately 300,000 kilometers per second; that is, 30,000,000,000 centimeters per second. Take the wave length as the 17,000th of a centimeter, and you find the frequency of the sodium light to be 510 million million per second. There, then, you find a calculation of the frequency from a simple observation which you can all make for yourselves.
Lastly, I must tell you about the color of the blue sky which was illustrated by the spherule embedded in an elastic solid. I want to explain to you in two minutes the mode of vibrations. Take the simplest plane-polarized light. Here is a spherule which is producing it in an elastic solid. Imagine the solid to extend miles horizontally and miles down, and imagine this spherule to vibrate up and down. It is quite clear that it will make transverse vibrations similarly in all horizontal directions. The plane of polarization is defined as a plane perpendicular to the line of vibration. Thus, light produced by a molecule vibrating up and down, as this red globe in the jelly before you, is polarized in a horizontal plane because the vibrations are vertical.
Here is another mode of vibrations. Let me twist this spherule in the jelly as I am doing it, and that will produce vibrations, also spreading out equally in all horizontal directions. When I twist this globe round, it draws the jelly round with it; twist it rapidly back, and the jelly flies back. By the inertia of the jelly the vibrations spread in all directions, and the lines of vibration are horizontal all through the jelly. Everywhere, miles away, that solid is placed in vibration. You do not see it, but you must understand that they are there. If it flies back it makes vibration, and we have waves of horizontal vibrations traveling out in all directions from the exciting molecule.
I am now causing the red globe to vibrate to and fro horizontally. That will cause vibrations to be produced which will be parallel to the line of motion at all places of the plane perpendicular to the range of the exciting molecule. What makes the blue sky? These are exactly the motions that make the blue light of the sky which is due to spherules in the luminiferous ether, but little modified by the air. Think of the sun near the horizon, think of the light of the sun streaming through and giving you the azure blue and violet overhead. Think first of any one particle of the sun, and think of it moving in such a way as to give horizontal and vertical vibrations and what not of circular and elliptic vibrations.
You see the blue sky in high pressure steam blown into the air; you see it in the experiment of Tyndall's blue sky, in which a delicate condensation of vapor gives rise to exactly the azure blue of the sky.
Now the motion of the luminiferous ether relatively to the spherule gives rise to the same effect as would an opposite motion impressed upon the spherule quite independently by an independent force. So you may think of the blue color coming from the sky as being produced by to and fro vibrations of matter in the air, which vibrates much as this little globe vibrates embedded in the jelly.
The result in a general way is this: The light coming from the blue sky is polarized in a plane through the sun, but the blue light of the sky is complicated by a great number of circumstances, and one of them is this: that the air is illuminated not only by the sun, but by the earth. If we could get the earth covered by a black cloth, then we could study the polarized light of the sky with simplicity, which we cannot do now. There are, in nature, reflections from seas, and rocks, and hills, and waters in an indefinitely complicated manner.
Let observers observe the blue sky not only in winter, when the earth is covered with snow, but in summer, when it is covered with dark green foliage. This will help to unravel the complicated phenomena in question. But the azure blue of the sky is light produced by the reaction on the vibrating ether of little spherules of water, of perhaps a fifty-thousandth or a hundred-thousandth of a centimeter diameter, or perhaps little motes, or lumps, or crystals of common salt, or particles of dust, or germs of vegetable or animal species wafted about in the air. Now what is the luminiferous ether? It is matter prodigiously less dense than air, millions and millions and millions of times less dense than air. We can form some sort of idea of its limitations. We believe it is a real thing, with great rigidity in comparison with its density, and it may be made to vibrate 400 million million times per second, and yet with such rigidity as not to produce the slightest resistance to any body going through it.
Going back to the illustration of the shoemaker's wax; if a cork will in the course of a year push its way up through a plate of that wax when placed under water, and if a lead bullet will penetrate downward to the bottom, what is the law of the resistance? It clearly depends on time. The cork slowly in the course of a year works its way up through two inches of that substance; give it one or two thousand years to do it, and the resistance will be enormously less; thus the motion of a cork or bullet, at the rate of one inch in 2,000 years, may be compared with that of the earth, moving at the rate of six times ninety-three million miles a year, or nineteen miles per second, through the luminiferous ether, but when we have a thing elastic like jelly and yielding like pitch, surely we have a large and solid ground for our faith in the speculative hypothesis of an elastic luminiferous ether, which constitutes the wave theory of light.
The weight of the conductors, says Henry Vivarez in La Lumiere Electrique, plays an important part in submarine telegraphy, not merely as a heavy item in the outlay, but as one of the principal factors in laying down the lines, and in taking them up in case of damage. When the conductor is being raised, the grappling-irons which lift it have to resist not merely the vertical component of the weight of the cable, but also the considerable effects resulting from friction against the water. It thus frequently happens, when working at great depths, that the conductor may be exposed to a strain greater than it is able to bear, and we are forced to have recourse to stratagems to bring it to the surface. These artifices consist in the use of two or more ships in raising, which is done as shown in Figs. 2 and 3, or, in the most simple cases, with the aid of an auxiliary buoy, as in Fig. 4. In any event, we see that the difficulties, and of course the cost of raising, must be considerable.
Hence to decrease the weight of the cables would be an important step in advance. If the weight is in general very great, it is because the copper core does not take any part in the strain which the entire cable has to resist. We know, indeed, that copper cannot bear a breaking-strain greater, at most, than 28 kilos per square millimeter. Besides, it would be elongated by such a strain by a very considerable fraction of its initial length; and, if the core were made to take part in any manner whatever in the strain which the entire cable has to support, it would be drawn out beyond its limit of elasticity, and would remain permanently elongated, while the substances in which it is inclosed would return to their natural length. It would result that, being no longer able to find room in a sheath which had become too short, the copper wire would take a sinuous form in its gutta-percha envelope, and would occasion at certain points ruptures, the effect of which would be to decentralize the wire, to perforate the layer of insulating matter, and finally to open out a fault in the cable.
But there exists an alloy (silicium bronze) which can be drawn out into wires having a conductivity equal to that of copper, and a mechanical resistance equal to that of the best iron. The use of this alloy would render it possible to set free the coating of the cables from a part of the strain which it now has to resist, and to diminish, consequently, their dimensions and weight. Wires are now made of this alloy, having a conductivity of from ninety-seven to ninety-nine per cent. of the standard, which at 0°C., and with the diameter of a millimeter, have a resistance of 20.57 ohms per kilometer. These wires do not break with a less strain than from 45 to 48 kilos. per square millimeter, and, which is a very precious property, their increase in length at the moment of rupture does not exceed one or one and a half per cent.
Let us consider the deep-sea section of cable of the French company from Paris to New York—the so-called "Pouyer-Quertier" cable, constructed and laid in 1879 by Siemens Brothers of London.
The respective weight of each of its component elements is, per nautical mile, copper core, 220 kilos; gutta-percha, 180 kilos; hemp, or an equivalent, 80 kilos; 18 wires of galvanized iron of 2 millimeters in diameter, 860 kilos; external hemp and composition, 400 kilos; total, 1,740 kilos. Total diameter, 30 millimeters. Total mechanical strength, 3,000 kilos, the wires of the covering being supposed to be of iron. Weight under water, 450 kilos. It can support its own weight without breaking for a length of from six to seven miles.
The Atlantic presents from north to south, and at about an equal distance from each continent, a sort of longitudinal ridge, in which the depths vary from 300 to 400 meters. This ridge spreads out, in 50° north latitude, into the region which has received the principal wires connecting England and France with the United States. On both coasts there are depressions in which the bottom is at the depth of from 4,000 to 6,000 meters. The one on the east extends from the south point of Ireland to the latitude of the Cape of Good Hope, and its left-hand boundary follows the general outlines of the west coasts of Europe and Africa. The two others, the northwestern and the southwestern, form two basins, bordering respectively on the United States and the Antilles and South America.
In these depressions soundings have shown certain zones in which the depths exceed 6,000 meters, the principal of which are found to the west of the Canaries, to the south of Newfoundland, between Porto Rico and the Bermudas, and to the right of the Isle of Marten-Vaz.
The great depths of the Pacific are differently distributed. Between Japan and California, between 40° and 50° north latitude, there is the Tuscarora depression, which has depths of from 6,000 to 8,000 meters. Parallel to Japan and the Kuriles there is a depression in which has been found the greatest known depth—8,513 meters.
We see, therefore, that any new great submarine line, having to extend into another zone than that which has received the present Atlantic cables, must traverse depressions in which the bottom reaches a maximum depth of 4,000 meters. The possibility of raising a damaged cable would be very problematical under such conditions, and it would become certainly impossible in case of a cable from San Francisco to Japan.
Under these conditions, we are forced to conclude that the use of the present cables limits strikingly the progress of submarine telegraphy, which must remain confined to certain zones of the Atlantic, to inland seas, and to lines along the coasts. But if we consider the daily progress of applied science, and the constantly increasing demand for rapid communication between nations, it is certain that we must shortly undertake the study of new cables intended to traverse the greatest depths of the ocean for long distances. Necessity, therefore, compels us to investigate the new solutions of the problem, which may furnish us with light cables, easy to lay, and possible to repair.
A cable made by Mr. J. Richards is composed as follows: core of silicium bronze equal in weight to that of the Pouyer-Quertier cable, or, per nautical mile, 220 kilos; gutta-percha, 180 kilos; layer of hemp, 80 kilos. The sheathing is formed of 28 wires of galvanized iron of 1.25 millimeters in diameter, each covered with hemp, and all twisted into a rope around the dielectric; the wires, 500 kilos: the hemp covering them, 250 kilos. The weight of the cable is, therefore, 1,230 kilos in the air, and 320 kilos in the water. Its diameter is 25 centimeters, and its resistance to fracture 2,800 kilos, of which the core supports one-half. Under these conditions, the cable can support from eight to nine nautical miles of its length, and can be raised from the greatest depths. The results of this comparative examination are self-evident.
For an equal conductivity and an approximately equal mechanical strength, the new cable is in weight and bulk equal to about two-thirds of the Pouyer-Quertier cable. It would cost about $165 less per mile, and would require, for laying, a ship and engines of less power, and therefore 7451 cheaper. The reduced armature will suffice to resist friction and the attacks of animal life in the deep sea; but for the shore ends we must keep to the types generally employed. Such as it is, and although it may undergo modifications in detail from a more complete study and from experience, it merits the attention of competent engineers.
Our adjoining engravings illustrate the system of J. S. Williams, for working electrical torpedoes, launches, and torpedo boats, and the appliances be proposes for their equipment and his method of utilizing a system of electrical appliances for the defense of sea-ports, harbors, coast, and coaling stations. We use Mr. Williams' own words in describing this invention. Fig. 1 illustrates men-of-war or vessels attempting to force their way into a harbor defended by such means. The movable and controllable torpedoes are indicated by letters of reference, A, connected through the medium of paying-out electrical cables, G, with the base of operations upon the shore at C, and the launches and floating torpedo batteries or vessels, D. Several lines of torpedo defense or attack are shown, and illustrate the hostile vessels coming within the destructive radius of the movable and controllable torpedoes, which radius is limited only by the length of the paying-out cable, which length can be 1½ miles (more or less). These means secure an effective weapon at all times under command from the base of operations over a radius of 1½ miles, as against a radius of 50 ft., which is the estimated effective range of destruction for fixed mines containing an equal explosive charge.
The movable torpedoes operated from the shore can be supplied with electric power from the main circuits extending along the coast from the developing source, at any distance from the electric power station or base from which the movable torpedoes are operated or supplied. Any natural force, fuel, or other means can be employed for the development of the electric force, which can be transmitted through the main circuits with high tension or pressure to the power stations along the coast, or to the floating magazines, where electric accumulators are placed to hold a reserve 7452 of energy. The accumulators at such stations can be compounded so as to be at all times ready for supplying power, and being charged, except when the limit of storage is reached. Electric cut-offs are provided in the loop or derived circuits from the main to cut the magazines out of the circuit when such predetermined limit of energy is in reserve, and means are employed to prevent the backward flow of the current toward the source from the power stations supplied from the main or other circuit. Means are also employed to automatically regulate and prevent any excess of current passing through the circuit in which the accumulators are included. The discharging circuits from the reserve magazines can be connected at the will of an operator with an electric circuit, including electric magazines, forming part of the equipment of the launches, vessels, or torpedoes, so as to supply electric power thereto. This can be accomplished at the wharves or through the medium of a cable buoyed along the coast, so as to obviate the necessity of the launches or vessels returning or running into harbor. Signaling devices can extend from such buoy to the operator along the shore, who will close the circuit from the reserve or main supply circuit. Fig. 2 illustrates a sectional elevation of an electrical torpedo provided with mechanism at the stern for operating the rudder electrically, and the force is regulated by an automatic or manually operative variable resistance interposed in the electrical circuit at the switch board of the cable. A circuit reverser and variable resistance are arranged upon the switch board, so that the operator at the base can change the direction of the current, and regulate the force applied through the medium of the electrical cable in such a manner as to adjust the rudder to port or starboard, and, if so arranged, to maintain it at any angle by varying the resistance in the circuit. The rudder mechanism can be operated by the electric energy stored on board the torpedo through the medium of an electric circuit thereto from the electric accumulator provided with a circuit closer and variable resistance worked by the force passed through the paying-out cable. The force passing there through is regulated by a pressure regulator and controlled by a circuit reverser and variable resistance upon the keyboard. Means are also employed for indicating to the operator the position of the rudder at any moment, and such position will correspond to some defined resistance introduced at any given moment in the circuit. The mechanism combined with the rudder can consist of an arrangement of compound solenoids, the armatures of which are connected to a lever on the rudder head, or a small electric motor can be employed for operating worm gearing in, or combined with, the rudder head. The rudder is brought back to the midship or normal position by springs or counterbalance weights.
The motor of the torpedo, as illustrated, is composed of a number of disk-shaped armatures fastened on the shaft, combined with the screw propeller; the field magnets, being also of disk form, are arranged so that the armatures revolve within close proximity, but not touching the pole surfaces. This enables an exceedingly high efficiency and great power to be realized from a motor of light weight. This construction of motor is specially suitable for use in the equipment of torpedoes and launches, and permits an increase of the power of the motor in either of two directions, i. e., either by increasing the number of disks of a given diameter upon the shaft, or by increasing the diameter of the disks, both of these methods giving increased power in direct ratio to the increase of size. The accumulator or secondary battery, c, is especially designed to store the energy in a small space, and with light weight, and so as to command an amount of energy representing the power necessary for a speed of 25 miles an hour or more. In the electrical circuit, between the motor and accumulator, variable resistances and other governing devices are interposed, by which the current passing to the motor is regulated automatically in accordance with the speed of the motor, or with the electric pressure in the circuit from the accumulator. A circuit closer or variable resistance operating in the circuit is connected by the cable with a variable resistance at the switch board, and operated by the current controlled thereby. The force to the motor can be regulated, controlled, or stopped at the will of the manipulator at the switch board placed at the point from which the torpedo is dispatched. Signaling devices or guide rods, O, for indicating the position and direction of movement of the torpedo to the operator can be arranged to be raised and lowered, through the medium of electrical appliances, P, at will, by a current sent through the paying-out cable from the keyboard at the base of operations. Fixed means or sight rods can be used, and hooded incandescent lamps, O2, can be carried by the signal or sight rods, by which means at night or in the day the operator will be enabled to direct the torpedo to the object of attack in spite of adverse or cross currents, or a change in the position of the vessel under attack.
The body of the torpedo containing the machinery and explosive can be arranged to be any desired depth below the surface of the water, and be supported by a buoy as a shield, or be covered by a protection against shot, the displacement of the torpedo being regulated in accordance with the means employed for maintaining it the desired distance below the surface. The torpedo can be ballasted and provided with fins to offer the necessary resistance to the action of the propelling machinery. The electrical paying-out cable, G, is shown in a coil in proximity to the chamber at the bow, which is designed to carry the explosive charge in a fixed or detachable magazine, arranged when detachable to drop a determined distance, and to be fired electrically by the operator or automatically.
Fig. 6 illustrates an apparatus in which a dynamo is operated by a rotary engine having a throttling device controlled electrically by the current passing through the discharging circuit of the generator; the circuit of the generator is connected with the paying-out cable of the torpedo, through the medium of the key board, in which a variable resistance and regulating devices are employed for controlling the operation of the torpedo. Electric magazines are shown arranged to operate in the discharging circuit of the generator, and to be connected with the appliances forming part of the equipment of the torpedo through the medium of the paying-out cable, in conjunction with which is arranged the circuit-closing devices of the switch board under the control of the operator at the stations. Automatic electric pressure regulators are used in the circuit from the source, so as to reduce or regulate the pressure to some predetermined limit. The circuit controllers and manually operative variable resistances upon the switch or keyboard can have indicators connected with them. Under such conditions, with the circuits and appliances upon the torpedo constructed to a known standard, the control of such torpedo in all its movements and operations is easy and certain. Such appliances are especially designed for use upon men-of-war or steam or electric launches when the torpedo vessels are not equipped with electrical magazines. Fig. 5 illustrates a floating fort or battery equipped with machinery, electrical apparatus, and torpedoes, as illustrated in Figs. 2 and 6. The floating fort or battery equipped with electrical or other machinery for propelling can be anchored in suitable positions, or moved from place to place to be in torpedo range of a fleet, or in a suitable position for supplying torpedo launches with torpedoes, and electric or other means of power.
Fig. 3 illustrates a steam launch, and Fig. 4 an electric launch fitted with electrical appliances and compartments containing a means for carrying and discharging electrical torpedoes. By the employment of such means, and a well-organized system of coast defense, it will be practically impossible for hostile vessels to land troops, or to inflict a serious damage upon shipping or seaport towns. Any extent of coast or estuary can be thoroughly protected by launches, light vessels, and appliances operated from fixed electrical stations, supplied with power and means of operation from any point, however distant. For carrying such a system into practical operation, the cost will, it is claimed, be but a tithe of what would be required for placing an inefficient system of fixed mines and forts, or for building men-of-war for coast defense, as men-of-war are practically defenseless against a greater number of high-speed launches equipped with movable and controllable torpedoes, the reasons for which are obvious, as a sufficient number of such launches would cover a greater distinctive range than the vessel which depended upon the range of its guns, or those combined with uncontrollable torpedoes.
Let not the epithet "Perpetual," which the inventor applies to the little apparatus that we are about to describe, frighten the reader, for its only purpose is to indicate that the instrument in question is capable of operating indefinitely, without care and without there ever being any need of taking it apart.
In this gas lighter the inflammation is produced by a small spark, but this latter, instead of being obtained by means of a pile, which, after a certain length of time, has to be mounted anew or entirely renewed, is secured by borrowing the energy produced by the operator pressing upon a button. It is, then, in reality, a mechanical lighter in which electricity intervenes as an intermedium charged with the transformation of work into sufficient of a spark to produce inflammation. Thanks to this principle, and to the arrangement of the apparatus, there is secured cleanness, safety, and economy.
The lighting is reduced, then, to opening the cock and placing the extremity of the rod over the burner, or over the edge of the glass in burners provided with a chimney. Upon pressing the button and then freeing it, a spark leaps between the two points and lights the gas. (Fig. 1).
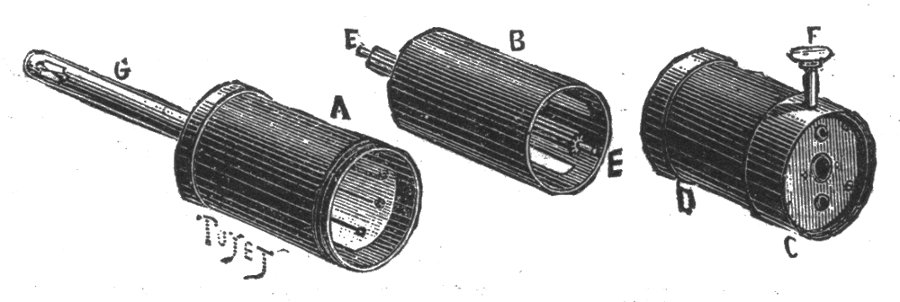
Fig. 2.—A, cylinder with lighting rod, G. B, movable cylinder fixed upon the axis, E. D, handle containing a rack actuated by a button, F.
The electric generator is a static induction machine of very small size, and the arrangement of which will be understood by reference to Fig. 1, which gives a general view of the apparatus with a portion removed in order to show the relative position of the different parts, and to Fig. 2, which shows the latter detached. A is an ebonite cylinder containing the entire machine, and closed above by a cap of the same substance upon which is screwed the lighting rod. The cap is traversed by conducting wires which end in two contact springs that establish an electric communication with the lighting tube.
Two inducting armatures of tin are cemented to the interior of the cylinder, A, and occupy, each of them, about a third of its circumference. The bottom of the cylinder, A, supports six contact springs, parallel with each other and constituting three distinct pairs which are properly connected, two by two, with the different parts of the rest of the apparatus.
The movable or induced cylinder, B, of ebonite is provided with six equidistant and insulated thin sheets of tin of a width nearly equal to the interval which separates them. This cylinder is given a rapid rotary motion by means of a system of rack and gearing every time the button, F, is pressed. During the revolution of the cylinder the six insulated plates come successively into communication with the six springs, and these put them successively in communication, two by two, first with the fixed inducting armatures, second, with the conductors connected with the two points between which the spark is to pass, and, third, with each other.
The apparatus operates, then, like Sir William Thomson's replenisher. It is only necessary for the armatures upon the cylinder, A, to be at the start at a difference of potential as small as desirable to suppose it, in order to have the play of the machine multiply the charge and soon give it sufficient tension to cross the interval that separates the two points fixed at the extremity of the lighting rod, G. From a technical point of view, the ingenious and new idea resides in the application of a multiplier of charges with which the priming and operation are always secured, provided the insulating parts are so dry that the losses due to dampness are inferior to the machine's power of production. This result, moreover, is easily attained by the use of a hermetically closed system, and of drying substances placed in that part of the cylinder which forms the handle of the apparatus.
From a mechanical point of view, the lighter contains a series of practical and simple arrangements which make it an apparatus at once convenient, strong, and sufficiently perpetual, as regards duration, to partially justify the name that has been bestowed upon it by its inventor, Mr. J. Ullmann.—La Nature.
In the accompanying cut we bring together a few figures of porcelain insulators for uncovered wires placed inside or outside of houses.
Figs. 1 and 2 represent simple and double channeled pulleys to be fixed against a wall, or upon a pole or a door post, by means of nails simply. Fig. 3 shows a pulley of larger dimensions for iron wires. Figs. 4, 5, and 6 show perforated insulators, that are quite convenient for holding and supporting a wire, but which are not convenient to put in position when the wire is of some length. Fig. 7 shows a device for protecting a wire that passes through a wall. Fig. 8 shows a support designed especially for small poles. It may be used either by passing the wires through the aperture or winding it around the neck of the bell. Fig. 8 shows a cleft insulator designed especially for fixing a wire in places where it must form an angle.—La Nature.
M. Brandt places alternately, in a continuous line, forty lamps of ordinary glass, forty of green glass, and forty of red glass, making a hundred and twenty lamps in all, at the foot of the stage. Each series of forty lamps forms a separate circuit. The three series can be lighted independently, or they may be combined, in order to obtain different effects of color. For example, a delicate rose hue may be produced by simultaneously lighting the red and the white lamps; a moonlight effect, by a combination of the white and the green lamps. In order to pass gradually from the latter to full daylight, it is only necessary to increase the resistance in the green circuit while strengthening the current in the white lamps. Moreover, the two sides of the stage may be lighted independently, because the 120 lamps are again subdivided into two circuits of sixty each. We may thus have a moonlight on one side of the stage, while the other side, at the moment when an actor enters with a torch in his hand, seems to be illuminated by the reflection from the torch. When the footlights are of gas, a current of hot air ascends above the whole line of lights, forming a sort of gaseous wall between the stage and the audience, which often makes it difficult to hear the actors. This inconvenience is suppressed by electric lighting, and the opera singers are agreeably surprised at the great improvement.—Lumiere Electr. 7453
It was not till 1867, on the occasion of the Universal Exhibition, that a dam was constructed at Suresnes that permitted of omnibus-boat service. The effect that this dam had was to raise the water 7½ feet up stream, and to consequently suppress the natural incline of the river between Paris and Suresnes. Its action made itself felt as far as to the Austerlitz Bridge in front of the Garden of Plants.
Between Suresnes and Lavallois the Seine is divided into two arms that are separated by the isles of Puteaux and Grande-Jabbe. The left arm was dammed at Suresnes, and here was established the sluice that allowed boats to cross the falls. The right arm was dammed at Levallois.
A law of April 6, 1878, decided the increase of the depth of the Seine between Paris and Rouen in order to allow boats of a draught of ten feet to reach Paris, and to bring thither, without transfer, English coal and Bordeaux wines. The Consul-General of the Seine having offered to contribute toward the expense, on condition that such boats might have it in their power to ascend as far as to Bercy, a law of July 21, 1880, decided that the Suresnes dam should be raised about three feet in order to increase the anchorage. To effect this, the dams of 1867 were entirely rebuilt, the new ones being located at Suresnes, across the two arms of the river. At the same time, the existing sluice was doubled by another one that was larger and deeper.
This great work was executed under the able direction of Mr. Boule, engineer in chief of roads and bridges, who has in charge the navigation of the Seine, outside of Paris, between Montereau and Poissy. The new sluice was constructed in 1880 and 1881, the dam to the left and the intermediate weir in 1882 and 1883, and the pass to the right in 1884. The width of the Seine at this point is about 820 feet, the length of the passes varies between 209 and 236 feet, and the two sluices occupy a width of 98 feet.
In the construction of the three passes there were established, up and down stream, dikes about 325 feet apart, thus giving considerable space for the installation of work yards, and much facilitating operations.
The new dam is closed by movable mechanisms of the kind invented by Engineer Poiret in 1834. The iron trestles that support the wickets are the largest that have ever been constructed, their height being nearly 20 feet and their weight 3,950 pounds. During freshets they are laid upon the bed of the sluice, and when the water subsides they are raised vertically. Upon these supports are placed swinging wickets, like those of mills, according to a system devised by Mr. Boulet in 1874, and which has been tried since then with success at the Port-a-l'Anglais dam near Paris. This system has likewise been successfully applied upon the Moskowa, below Moscow, and upon the Saone, at the Mulatiere dam, near Lyons.9
The construction of the new sluice presented great difficulties, by reason of the fact that it was necessary to avoid obstructing navigation in the existing sluice, where the boats stood thirteen or fifteen feet above the laborers who were working at the side, behind simple dikes. Yet it became necessary to forbid the passage of the sluices for nearly a month each year. At Suresnes this was taken advantage of each time to keep the works in full blast during the whole night, the lighting being done by electricity. During these interruptions the boats accumulated at the sides of the dam, and gave the public an idea of what Paris would be as a sea port.
All the work is now finished. Its estimated cost is six millions, two of which were devoted to the construction of about half a mile of dock wall and of a long and wide sewer.
The sluices were opened for navigation on the 15th of September last. The new dams will be in operation in 1885, and next summer they will increase the height of water in Paris by one meter.—L'Illustration.
Mr. Fred. W. Brearey has been the honorary secretary of the Aeronautical Society of Great Britain ever since its establishment in 1866. In the course of his experiments, extending over some years, he found that if a serpentine action were imparted to a fabric it would propel an attached object many times its weight in the air. He records in his published magazine articles that he took the idea from watching the movements of a skate in an aquarium, which in swimming undulated its whole body.
In applying the principle to locomotion in air, it is of course impossible to undulate what may called the backbone of the whole structure in the manner of the skate. But a fabric may be so attached to a receptacle, and so worked from thence by a suitable motive power, that its undulations will propel and support a considerable weight, depending upon the energy with which such fabric is thrown into waves. He believes that the awning of a vessel can be made in this way to contribute to a ship's progress at the same time that it would cool the passengers.
Mr. Brearey argues that the instinct of the bird enables it to adapt itself instantaneously to varying circumstances; that in any arrangement for effecting flight by machinery—the adjustment of parts to meet sudden requirements being a matter requiring momentary thought—it is desirable, if practicable, to employ large surfaces for parachutic action, at the same time making this means of safety not an incumbrance, but an aid. The possession of instinct allows of the employment of the smallest surface in proportion to weight; the possession of forethought renders it necessary that intermittent action shall be safeguarded by large surfaces.
This requirement is fully met, the inventor says, by the arrangement advocated by him, and none but edge resistance is offered to the air, except the sharp lines of the necessary vehicle. The manufacture of such an apparatus upon a scale of utility would be as follows:
A flat-bottomed receptacle, somewhat of boat shape, would be fixed upon wheels. At the fore part of the boat a motor would from each side elevate and depress two wing-arms, each 15 ft. long. (See Figure.) Along the wing-arms is attached a fabric which would form the front part of a kite, which, being fastened in the center to the edge of the boat, would continue for 15 ft. to the rear, being extended about 6 ft. farther than the stern of the boat by a continuing spar. To a cross piece here would be fastened the tail end of the kite, which, however, instead of a point, would be about 5 ft. in width. From this again would extend a tail of about 12 ft., to which either a lateral, twisting, or a vertical movement could be imparted by cords in the hands of the operator in the boat for steering purposes. From the fore part of the boat would extend a bowsprit, from which cords would be attached to the two wing-arms to prevent the weight of the fabric from dragging them backward.
An important arrangement has been adopted by the inventor, which he calls the pectoral cord, which by its automatic action assumes the functions of the pectoral muscle of the bird. This is an India-rubber cord. It is attached by its two extremities to the under portion of each wing-arm, and in models passes underneath a central shaft—in this case the boat. Its degree of elasticity is regulated by the weight. When any model with wings is committed to the action of the air, the pressure of the air causes the wings to fly upward, and power is required according to the weight sustained to depress the wings against the weight. The strength of the cord, however, is such that it maintains the outstretched wings at that angle which is suitable for gliding upon the air without, in the case of the bird, any enforced muscular exertion. The contraction of this cord assists the power exerted in the downward stroke.
The wing arms would not be rigid throughout their length. They would consist of a number of rattans or canes firmly bound together by close wrapping, and tapered by cutting 7454 off one at intervals, this being practically unbreakable by any accident likely to occur. The portion next to the body for 5 ft. or 6 ft. might be stiffened by a steel tube, forming the center round which the rattans are wrapped. By this method of forming the wing-arms their length may be increased at pleasure.
A small model upon this principle, but without any motive power, was liberated as an experiment by Captain Templer, from a balloon which had risen 200 ft. or 300 ft. from Woolwich Arsenal, and it traveled back again to the arsenal half a mile against the wind uninjured.
The importance of such an apparatus might become manifest in any flight of a balloon from a besieged place over the heads of an investing army. The results of a rapid survey of the enemy's positions could be written and dispatched from a height against the same current which wafted the balloon, so as to fall within the lines of the besieged.
Given a light motive power, which it is hoped may soon be forthcoming, Mr. Brearey anticipates the action of the machine as follows:
A surface will be provided according to the weight to be carried, the supporting surface of a parachute being known. Upon being run down an incline the envelope will be inflated by the pressure of the air, and the wing arms raised to that point where their further elevation is restrained by the pectoral cord. The machine will then naturally float away from the incline, and the occupant must set his motor in action. The downward blow of the wing-arms will cause the fabric immediately attached thereto to imprison a mass of compressed air, and the following wave will force it along the under side of the fabric. This will cause propulsion.
The return or up stroke cuts off and diverts from the upper part that air which, but for the rise of the wing-arms, would flow over the back, and shunts it underneath, while that which is embraced in the concave fabric following the up-stroke is thrown off in a wave to the rear above the machine, and so on alternately.
During this energetic action the whole fabric is kept in a state of corrugation, and to such extent is rigid. It possesses all the properties of a plane, and superiority over a plane, inasmuch as it propels itself, and upon cessation of action assumes the functions of a parachute, the descent of which a man may regulate by a step backward or forward.
The latest invention which has been completed upon a full scale is the idea of Mr. H. C. Linfield, of Margate. It is really a plane-propelling machine, but the planes are compressed, it may be said, into small compass, being only two inches apart, and being of such number and extent as to present 438 square feet of strained and varnished linen in two frames, each five feet square. The dimensions of the machine are 20 ft. 9 in. in length, 15 ft. in width, and 8 ft. 3 ins. in height. It runs upon four wheels; the two front wheels are 6 ft. in diameter, the two hind wheels 3 ft. The frames before mentioned are fixed one on each outer side of the front wheel at an upward angle. The wheels have been tested to sustain a weight of 5 cwt.
The weight of the machine is 240 lb., and of its inventor 180 lb. He sits between the wheels and works two treadles, which actuate a nine-bladed screw 7 ft. in diameter, fixed in front of the machine, to which he can impart 112 revolutions per minute. This suffices to enable him to travel along a level road.
During the erection of the viaduct at Douarnenez—Department of Finistêre—over the river Pouldavid, one end of one of the heavy latticework girders dropped into the river, as shown in the upper one of the annexed cuts taken from L'Illustration. The difficult problem to be solved was to remove the obstruction in as short a time as possible, and at the least expense; and the engineers came to the conclusion that it would be best to raise the fallen end, as the girder was intact, with the exception of those parts that struck the bottom of the river, and which could easily be replaced by others.
The viaduct has three spans of 190 ft. each, and is 88 ft. above the surface of the water. While rolling the girders upon the piers, the pivot of one of the rollers broke, and a projecting length of 183 ft. of the girder dropped a vertical distance of 72 ft. That part of the girder that had to be raised was 183 ft. long, and weighed 145 tons, and the free end had to be moved a distance of 72 ft. in an arc the radius of which was 183 ft. Suitable scaffoldings were erected on the piers and below the fallen end of the girder; four strong and heavy double chains were connected with the lower end of the girder and passed over a scaffolding erected for this purpose, and the opposite ends of the chains were connected with a heavy box weighted with rails, and containing 2,700 cubic ft. of water. The upper end of the fallen girder was disconnected from the other parts of the structure, and a heavy steel pivot bar inserted, upon which the girder could turn. The box was so weighted that the fallen girder was somewhat heavier than the box, and then windlass chains were connected with the lower end of the girder, and wound upon windlass drums operated on top of the scaffolding. The weighted box thus merely acted as a counterbalancing weight, the raising being accomplished by means of the windlass. On the 1st of August the lower end of the girder was raised 17 inches, and remained in this position for twenty-four hours, during which time examinations were made which proved that the calculations were correct, and that all the parts worked perfectly. The operation was completed the next day with perfect success, and was witnessed by a great multitude, attracted by the novel sight.
The illustration represents a multiple wire tester, constructed for the Trenton Iron and Steel Company by Riehle Bros., of Philadelphia. It consists of a weighing mechanism (seen on the left, with a capacity of 4,000 pounds), two single or alternating pumps, a hydraulic jack, a patented three-way valve, and a rising and falling accumulator.
The weighing end of the machine, placed horizontally and secured by bolts to a foundation, is accurate, and will weigh the strain on one to six wires at a time. It is provided with self-adjusting grips to take in wires from No. 10 to No. 16, and hold them firmly. It can be adapted to take in a larger or smaller range of numbers when desired. There is a set of gripping appliances at both ends, and in the present instance they are 90 feet apart—one set at the scale end, and the other secured to head of piston. The jack is 5 feet in length, and lined with brass; its outside diameter is 3½ inches; its inside diameter, 2¼ inches. Like the scale end, it is firmly bolted down to its foundations.
The plunger has a stroke of 4 feet. It is supported and guided by three guides, the top one being a straight tube running on turned rollers. A three-way valve controls the movements of the jack and accumulator, and supplies water to the jack by a lever. When the lever is raised, the water is forced into the larger area of the jack, causing the plunger to move backward and bring a strain on to the wires or other specimens; when the lever is lowered, the water in the larger area of the jack only returns to the reservoir of the pump (to be used again). Now, without changing the position of the lever, the plunger will return automatically, without weight or counterbalance, with a steady, smooth, and uniform motion.
The pump has a slow motion, 60 revolutions per minute. It has two single action pistons, and the valves are so simple and readily accessible that an ordinary mechanic can examine and repair, when necessary, in a short time. The accumulator is so arranged as to overflow when it comes to its maximum height. The machine can be adapted to stretching and straightening wires in lengths to a given amount.
The weight on the scale and that on the accumulator is made to correspond, so that wires of a certain number or size can be quickly tested in quantities under exactly the same conditions, with only the movement of the lever.
The tenacity with which whip cord, cotton cord, and other similar lines preserve their twist when properly made, is a little remarkable when considered in relation to the materials from which they are manufactured, and which as a rule show a tendency when ordinarily twisted to return to a straight line. This was one of the reflections which occurred to us when watching a James doubling and laying machine at work at the late Textile Exhibition, on the stand of Walter T. Glover & Co., of Manchester. We give a perspective view of this machine.
There are several ways of carrying out the process of doubling, which in its simplest sense consists in laying a given number of folds of yarn together and putting a twist into them. But beyond this we come to spindle banding, which is cord, or rope in miniature, and it is made of three or more ends of the doubled yarn just mentioned, such doubled yarn becoming, in fact, the strand of a small rope. To lay these strands properly into a cord they should not only be twisted together, but each should be twisted separately in 7455 the opposite direction to the twist of the cord. A banding machine, therefore, has to impart a double twist, and to perform the work perfectly each twist should be capable of easy regulation; and the drag upon the bobbins should admit of being adjusted to requirements. These conditions are met in the James machine, as evidenced by the samples of work produced by it. As shown in our engraving, the apparatus consists of three heads, of four spindles each, being capable therefore of doubling a four-strand cord. The heads work independently of each other, and by throwing one or two of the spindles out of action a three-strand or a two-strand cord will be produced. The cord is twisted regularly, and may be made continuously to any length. The uniformity of the twist depends upon the fact that the cord is taken up at a regular rate, by a simple and neat motion, consisting merely of a pair of pulleys, one grooved and the other with a roughened surface. After leaving these, the cord is coiled upon the reels seen on the top of the framework. The twist given to the cord depends upon the rate at which it is taken up, the speed of the center spindle remaining constant. The twist in the strands is governed by the speed at which the bobbin spindles revolve. This may be adjusted as required, by a series of change wheels. An effective stop motion is also applied to automatically stop the head in which a breakage takes place, whether of the cord itself or of a single strand. Either head is started by depressing the handle or knob in front of it. A feature for which particular merit is claimed is that a heavy drag is put upon all the strands separately for the purpose of taking out all the stretch before twisting, which is an important desideratum for the production of good banding. This is accomplished by hanging weights on the spindles, which cause the strands to be twisted under tension. The tension is altered as required by the size and nature of the yarn, by removing or adding to the weights. The heads being independent of each other enables the machine to be employed on three cords of different material and thickness. The production is about 1,300 yards per head for ten hours, and it is stated that a girl can mind as many as 80 or 90 heads.—Iron.
The following table gives the draught area and heating surface of the various sized boiler tubes and flues:
| External Diameter. |
Draught area in sq. inches. |
Draught Area in sq. feet. |
Heating surface in feet per ft. of tube in length. |
No. of tubes in 1 sq. foot of draught area. |
|---|---|---|---|---|
| 5/8 | ...... | ...... | .1636 | ..... |
| ¾ | ...... | ...... | .1963 | ..... |
| 1 | .575 | .0040 | .2618 | 250.0 |
| 1¼ | .968 | .0067 | .3272 | 149.3 |
| 1½ | 1.389 | .00964 | .3927 | 103.7 |
| 1¾ | 1.911 | .0133 | .4581 | 75.2 |
| 2 | 2.573 | .0179 | .5236 | 55.9 |
| 2¼ | 3.333 | .0231 | .5891 | 43.3 |
| 2½ | 4.083 | .0284 | .6545 | 35.2 |
| 2¾ | 5.027 | .0349 | .7200 | 28.7 |
| 3 | 6.070 | .0422 | .7854 | 23.7 |
| 3¼ | 7.116 | .0494 | .8508 | 20.2 |
| 3½ | 8.347 | .0580 | .9163 | 17.2 |
| 3¾ | 9.676 | .0672 | .9818 | 14.9 |
| 4 | 10.93 | .0759 | 1.0472 | 13.2 |
| 4½ | 14.05 | .0996 | 1.1781 | 10.2 |
| 5 | 17.35 | .1205 | 1.3090 | 8.3 |
| 6 | 25.25 | .1753 | 1.5708 | 5.7 |
| 7 | 34.94 | .2426 | 1.8326 | 4.1 |
| 8 | 46.20 | .3208 | 2.0944 | 3.1 |
| 9 | 58.63 | .4072 | 2.3562 | 2.5 |
| 10 | 72.23 | .5016 | 2.6180 | 2.0 |
We give below two views of a ladle carriage which has been constructed from the designs of Mr. Thomas Wood, the chief engineer to the Ebbw Vale Steel, Iron, and Coal Company. These works cover a large extent of ground, the Victoria furnaces and the Ebbw Vale furnaces, both of which supply one steel plant, being over a mile apart. Although this gives a long distance over which the molten metal from the furnaces has to be carried, it is by no means unprecedented; the Barrow furnaces for instance being situated still further from the steel works they supply. Until a short time ago, however, the Ebbw Vale Company had their Sirhowy furnaces in blast. These are, or rather were, for now they are dismantled, situated six miles by rail from the converters they supplied at Ebbw Vale, consequently the ladle containing the 10 tons of molten metal had to be brought this distance each time the converters were charged. In order to meet the exigencies of such a service, the ladle carriage we now illustrate was designed by Mr. Wood.
By means of the gearing of wormwheel, rack, and pinion, which are clearly shown in Fig. 2, the ladle can be retained in the center of the carriage and kept upright for running; a clip which is easily knocked out of gear being fitted to retain it in the necessary position. When the ladle is in the required spot to enable the charge to be tipped into the runner which takes it to the converter, the loose wrought-iron handle, A, is slipped on to the square end of the wormshaft, and by turning this the ladle is tipped, and at the same time travels on the rack from its position in the center of the carriage, one man being sufficient to perform the operation. The dotted lines at B represent a wrought-iron shield for protecting the tipping gear from splashes of metal, etc.
With the old cast-iron frame carriage the weight of the ladle and charge is practically carried by the two bearings on one side, as the ladle has to be overhung from the center of the carriage, in order that the metal may tip clear of the rails and into the well; supposing of course there are not conveniences for tipping direct into the converter.
It will be seen that in Mr. Wood's arrangement, when the ladle is in a vertical position it stands fairly in the middle of the carriage, but the action of tipping carries it to the side, so that the charge will clear the rails. This carriage has now been in work for about three years, and since its introduction there has not been the slightest hitch, even when running ten tons of metal at a considerable speed over the six miles of line from the Sirhowy furnaces. This has been a pleasing contrast compared to the trouble that used to be experienced at Ebbw Vale with the original cast-iron frames. These, under the heavy duty put upon them, were continually breaking on the side which had to carry the weight, and this would entail the metal having to be tipped on the ground so that it might be broken for recharging.
Although the exceptional nature of the work at Ebbw Vale called forth this arrangement, it will of course be understood that the advantages it possesses are also manifest upon shorter journeys.—Engineering.
The tubes of tubular boilers must, for different reasons, be taken out when the generator has worked for a certain length of time. Such a necessity presents itself when the extremities of the tubes are worn out and can no longer be fastened with sufficient tightness into the plate, or when the portion of the tube in contact with water is so incrusted that there results a notable diminution in the production of steam, or when the tubes exhibit local injuries, or, finally, when the interior of the boiler must be examined.
This latter contingency arises for every boiler after a period of from 6 to 8 years, and it requires the removal of all the tubes. It furnishes an occasion to remedy the other defects, that would have of themselves required the renewal of only a certain number of the tubes. In the interval between these thorough inspections defects may present themselves which require the removal of a certain number of tubes. The frequency of such repairs depends upon the nature of the feed water, upon the quality of the fuel, upon the pressure at which the generator operates, upon the state of repair in which the boiler is kept, and naturally also upon the quality of the metal of which the tubes themselves are composed.
Selenitic water deposits in the long run a very hard and adhesive incrustation, which acts as an obstacle to the transmission of heat.
The more calcareous waters fill the intervals between the tubes with deposits which can be but partially removed by the washing of the boiler, and which often form a calcareous mass such as to prevent all circulation of water around the tubes.
In both cases the tubes are heated beyond measure, elongate, detach themselves from the tube-plates, and burn in places, or lose enough of their resistance to allow them to become flattened by the pressure of the steam.
The loosening of the tubes likewise acts injuriously upon the plates, which the pressure causes to bend outwardly. The result is that the tubes may become completely detached.
Sulphurous fuel corrodes the extremities of the tubes near the fire-box and also notably attacks the hind extremities, in the interior, against the tube-plate. It likewise renders brittle those tubes whose metal is bad, so that they split either of themselves or at the least effort made to tighten them up in the tube-plate.
In tubes made of poor metal these brittle places are not only found near the plates, but also in other parts.
The tubes likewise have to undergo too lively a combustion when the boilers are driven. Leakages from the tubes often proceed from the fact that an expansion of the boiler lengthwise is prevented, or from a cooling of the tubes by a current of air which passes, without becoming heated, through a badly covered grate. Leakages may also occur if a boiler that has just been emptied is filled too soon.
It will be seen that the causes of the deterioration of tubes are numerous; and the repairs that they give rise to in railway shops are therefore very important, and are generally known as a whole. Yet they differ in some points of detail according to the shop in which they are made, so that it may not be without utility to pass them in review, in order to compare the results of the practice of several persons pursuing the same object.
The author of this article has had, during a long experience, occasion to make such comparisons: several of the methods that he describes were derived by him in shops that he directed, and have been applied upon a large scale; and numerous visits to other shops have permitted him to see different processes and to judge of results.
The different repairs to be made in boiler tubes may be classified as follows:
1. Repairs to leaky tubes.
2. Removal of worn-out tubes.
3. Repair of tubes in service, and putting them in place again.
Leakages at the point of insertion of the tubes are still generally and exclusively repaired by means of roller apparatus for opening the tubes, and with which an endeavor is made to tighten the latter in the hole in the tube-plate.
The cones which were formerly employed injured the ends of the tubes by splitting them; if the workman was not very skillful, the holes in the plate became oval; and fractures likewise quite often occurred between the holes in the plate itself.
The best apparatus to open the tubes are those in which the wedge that separates the rollers is actuated by a screw; those in which the wedge is driven in by a hammer are scarcely better than the old cones.
When apparatus for opening tubes are used, care must be taken to begin with the external tubes, and to open these gradually. The same operation is afterward performed upon the neighboring ones, in approaching the center, and then the first ones are taken up. The tubes should never be opened at one operation, but each one should be subjected to several passes.
At the second pass, the rollers should be placed a little more deeply, and should then be half within the tube-plate. The tube thus opens behind the plate and forms a bearing against it, and this not only renders it tighter, but also increases 7456 its adhesion to the plate. Finally, the operation is finished by beating down the edge of the tube that has been raised a little by the preceding pass. If this edge is already somewhat deteriorated, or if it is not very thick, tightness may be had by means of rings. The use of rings should be avoided as much as possible, because they diminish the section of the tubes, and render the cleaning of their interior more difficult. They should only be employed as an exception, and should be considered as an unavoidable evil. Even in old boilers, in which the holes have become oval, they should be considered only as a means of rendering a small number of tubes tight.
The tubes are taken out independently of one another through the front tube-plate, after an incision has been made with a chisel through the part of the tube that is fixed into the back plate. When the holes in the front tube-plate are not greater in diameter than the external diameter of the tube, and the latter is incrusted, this process becomes very difficult, and the use of it often completely spoils the tube. In fact, we can only remove the tube by live force, and for this purpose we either use the shock of a heavy body or mechanical apparatus upon whose arrangement I shall not dwell.
In all cases the holes of the tube-plate are injured. The edge of these must, in fact, detach the scale from the tube before the latter can be removed from the boiler, and, when a little of this scale remains adherent, it produces grooves in the hole, which render it very difficult later on to make the new tube tight. It is consequently preferable to cut the tubes immediately back of the plates by means of a special apparatus consisting of a cone provided with a small circular steel saw.
This operation should be begun at the bottom of the boiler near the blow-off plug, and be continued in advancing toward the top. The cut tubes fall to the bottom of the boiler, and are removed through the blow-off hole of the front tube-plate. The pieces of tube that remain in the plate are afterward easily removed by cutting them with a chisel.
If there are but few tubes to be removed, a passage is made for them toward the blow-off plug by removing a few of the tubes beneath. When the tubes to be removed are not too far from the plug, this method is very satisfactory. Even though there were a few more tubes removed, the cost of such removal would be more than compensated for, because this method is cheaper, and preserves the tubes and plates, and because the boiler, by receiving a larger number of clean tubes, will afterward utilize the fuel better.
Either when the removed tubes are to be employed anew, or when they are to be classed as old material, it is equally necessary to free them from the incrustation that covers them. The methods employed vary according to the shop.
The cleaning of tubes by beating or scraping the incrustation is very difficult, and requires much time. In some shops the tubes are dipped into an acid bath. In this way only the incrustation composed of carbonate of lime is dissolved, that into the composition of which sulphuric acid enters not being attacked.
In some large shops there are iron drums in which the tubes are placed. When these drums are revolved the incrustation becomes partially detached, but very rarely completely, and it is always necessary to finish the work by hand. It also happens that the bits of scale that become detached and that remain between the tubes produce grooves therein; besides, the cost of installing these drums is quite high.
Per contra, the writer has seen a, as yet, little known method employed in the shops of the Berlin-Hamburg Railway, one that he has used himself, that he has introduced into several shops, and that he can recommend as the best.
The tube to be cleaned is submitted to a rotary motion around its longitudinal axis. The workman grasps it with a sort of wooden pincers whose jaws are provided with coarsely toothed steel plates, and, pressing the legs of this more or less tightly, slides it slowly along the tube. The incrustation is thus reduced to dust, and the tube, after the operation, is absolutely clean.
The apparatus used for revolving the tubes is shown in Figs. 1 to 3. It consists of quite a short shaft, which revolves in two pillow-blocks and receives its motion through pulleys. Outside of the bearing to the right, this shaft terminates in a cone provided with channels whose diameter is proportioned to that of the tubes.
The tube to be cleaned is firmly fixed upon the cone, and provided at its other extremity with a plug that serves to center it. As the cleaning is accompanied with much dust, it must be done in open air or in a special shop.
At the same time, a classification is made of the damaged tubes that can no longer be employed, except as ends of the tubes that may be employed in shorter boilers, and of those that are entirely unserviceable.
Every time a tube is removed it loses 70 to 80 mm. of its length in the two cuttings. When we have locomotives that are provided with shorter boilers, we have a direct use for the removed tubes, but if the contrary is the case the tubes must be lengthened. Such elongation is effected in three ways, viz., by drawing them out, by soldering copper ends to them, and by uniting iron ends to them with a hammer.—F. W. Eichholz, in Organ fur die Fortschritte des Eisenbahnwesens.
The French Agricultural Machínery Company has recently made a very interesting application of the screw for raising water for submersion and irrigation, and, to our knowledge, it is the first of its kind.
It is only necessary to examine the accompanying cut and observe the dimensions of the machine (which was constructed according to plans of Mr. Grulet) to recognize the fact that we have here a really practical application.
The screw, which constitutes the principal peculiarity of the system, has six blades, with a pitch of 0.465 m. On making 210 revolutions per minute it is capable of raising about 435 liters (95 gallons) per second to a height of 1.2 m (about 4 feet). The shaft that drives it revolves in a bearing which is bolted to a cross piece that is affixed to the cylindrical chamber. This latter consists of a cast iron case that is easily taken apart, and of a strong cylinder of iron plate whose upper extremity is connected, by means of riveted angle iron, with the bottom of the sluice. In the interior of the cylinder there are two cones, whose bases embrace the hub of the screw in such a way as to obtain a continuous superposition of the layers of liquid, and prevent bodies in suspension from penetrating between the rubbing surfaces of the bearing. One of the cones is made of iron plate, and is connected with the principal cylinder by four radiating braces and small angle irons, and the other is cast in a single piece with the box of the pivot.
The rotary axis is guided above by two pillow blocks held by the cross pieces of a frame that is riveted to the sides of the sluice. Finally, this latter terminates in a hinged gate which regulates the flow of the water.
Two beams that rest upon the sides of a stream will suffice in most cases to support the entire affair.
The mechanical duty of the apparatus is estimated at about 65 per cent. In the apparatus put up by Mr. Grulet, the motive power is furnished by a portable 10 H.P. engine. The boiler is a return flame one, with movable fire place, and the steam cylinder has a diameter of 0.2 m. (8 inches) for a piston stroke of 0.3 m. (about 12 inches). Before the apparatus was finally put in place it was sent to the last exhibition at Carcassonne, where it attracted very much attention from visitors. Its great regularity in working was particularly remarked. This quality, and the simplicity of its construction and the ease with which it may be put in place, are valuable features in apparatus that are designed to be looked after by inexperienced persons, and to operate in open air far from repair shops.—Revue Industrielle.
In alkaline toning with borax or acetate of soda, the first consideration is to free the paper as much as possible from the excess of silver nitrate remaining therein over and above the quantity used in the production of the print; this is termed washing away the free silver. That operation is satisfactorily performed by soaking the prints in a few changes of clean soft water, usually four, or until the water is no longer opalescent when tested with a few grains of salt. The washing water so obtained is collected in the manner described to you by Mr. F. W. Hart, and precipitated with dilute hydrochloric acid. The vessel employed should be scrupulously clean, either earthenware, porcelain, or wood answering the purpose.
Experiment 1.—The treatment of the prints is sometimes followed by passing them into a dilute solution of sodium acetate or ordinary common salt, about one per cent., such as here shown, and stirring them about for five minutes, when it will be seen they have assumed a brick-red color, the object of which is threefold: First, the fibers become charged with a substance which acts as a chlorine absorbent, a necessary property to be mentioned further on. Secondly, a definite color is insured to start with, thus obviating the possibility of mistaking fresh prints in the toning bath for those which have become purple by reason of the deposited gold, an important consideration when dealing with fumed paper. Thirdly, the last trace of free nitrate of silver is removed, thereby preventing a too rapid decomposition of the toning bath.
Theoretically considered, it is proper that the last trace of silver nitrate should be removed; but those who are engaged in the daily practice of commercial work do not insist upon the strict observance of such a rule in all cases. An especial exception is permitted and advocated when dealing with prints from weak or underexposed negatives, this class being found to yield richer tones by not washing any of the free silver out.
The plan of soaking prints in a solution of sodium acetate was originally recommended, in lieu of washing, by a member of this Association, Mr. A. L. Henderson, as long ago as 1861, the following being an outline of the method suggested by him: Slightly overprinted proofs were soaked in a bath composed of
| Sodium acetate | 240 | grains. |
| Water | 10 | ounces. |
The unwashed proofs were moved about in this solution at least ten minutes, in order to convert all the free silver nitrate into acetate of silver. After slight rinsing in clean water the proofs were toned with
| Gold terchloride | 4 | grains. |
| Sodium acetate | 240 | " |
| Water | 10 | ounces. |
Among the advantages claimed was an entire absence from mealiness, a defect, you will remember, we now avoid by the adoption of ammoniacal fuming.
Guide-books to the practice of printing usually recommended three rapid washings; the decomposing action thus set up by the quantity of free silver remaining in the paper materially quickens the speed of toning. To prevent a too rapid deposition of gold some printers prefer adding a small quantity of common salt to the toning bath, which turns the prints sufficiently red and acts in some respects equal to an intermediary bath.
Preserved papers—containing, as they generally do, a certain proportion of free acid—are liable to give some trouble in toning, owing to the retarding action of the acid present. When this occurs, it is in a great measure overcome by the use of an intermediate bath of an alkaline character and sufficient strength to neutralize the acid. Either the carbonates of ammonia or soda are found useful for this purpose, and I cannot do better than quote the one mentioned by Mr. Frederick York, which, it will be remembered, is composed of
| Washing soda | 1 | ounce. |
| Water | 1 | gallon. |
Prints treated in the manner described are ready for toning by the alkaline method to be dealt with later on.
This brings us to the consideration of toning baths generally. The properties of toning baths vary somewhat according to the mode of preparation. The term "toning," as we understand it, implies a certain change of color brought about by chemical means, such as the deposition of a stable metal upon one that is easily affected by the atmosphere—electrolysis, in fact.
Evidently Mr. W. H. Fox Talbot was the first to use the toning bath in connection with paper photography, although he does not seem to have made much headway with his process at first; for it is recorded that from January, 1839, the date when Mr. Talbot communicated his discovery to the Royal Society, until 1845 very little improvement took place. These early paper pictures, be it remembered, were designated "photogenic drawings." Talbotype was not patented for some time afterward.
In the year 1845, however, it was found that steeping the paper in terchloride of gold vastly improved the results. It was not until 1853 that albumen took any part in the production of prints, the honor of its introduction being ascribed to Mr. Henry Pollock, although it seems that M. Le Gray, of Paris, about that time was producing stereoscopic pictures on albumenized paper. To M. Le Gray is due the credit of introducing gold toning in lieu of sulphur. The first toning then was performed by the decomposition of hypo., and known as "sulphur toning," by which fine black tones were obtained upon the addition of an acid, such as acetic, sulphuric, or other suitable oxidizing substance to the hypo., gold taking no part in this process. Unfortunately, prints so treated are said to be the least permanent of any; but of that I can bring no actual proof, never having employed the process.
Experiment 2.—Toning by Sulphur.—We have an unwashed silver print here in a twenty per cent. solution of hypo., and to that we now add a few drops of slightly dilute sulphuric acid. It will be seen that a straw-colored substance is immediately liberated, which is sulphur in an exceedingly fine 7457 state of division, and this becomes attached to the print. Toning action goes on, through the silver image being tarnished, or, more correctly, converted into sulphide of silver. This liberation of sulphur may be expressed by the following equation:
| Hypo. | Sulphuric Acid | ||
| Na2S2O3 + | H2SO4 = | ||
| Glauber Salts. | Water | Sulphur Dioxide | Sulphur. |
| Na2SO4 + | H2O + | SO2 + | S. |
With respect to the reaction which takes place when toning a silver image with sulphur, I will quote a few lines from the parent work of reference for nearly all recent writers, namely, Hardwich's Photographic Chemistry, wherein we find the following paragraph:
"It is well known that articles of silver plate become darkened by exposure to the fumes of sulphur, or to those of sulphureted hydrogen, of which minute traces are always present in the atmosphere. If the stopper of a bottle of sulphureted hydrogen water be removed, and a simply-fixed photographic positive suspended over it, the picture will lose its characteristic red tone, and become nearly black. The black color is even more intense than an experienced chemist would have anticipated, because analysis teaches us that the actual quantity of silver present in a photographic picture on paper is infinitesimally small; and it is well known that sulphide of silver, although of a deep brown color, approaching to black when in mass, exhibits a pale yellow tint in thin layers, so that a mere film of silver converted into sulphide possesses very little depth of color. To explain the difficulty it has been suggested that the toning action of sulphur on a red print is probably due to the production of a sub-sulphide possessing an intense colorific power, like the sub-oxide and sub-chloride of silver. When the toned picture is subjected to the further action of sulphur, is converted into the ordinary protosulphide of silver, and becomes yellow and faded."
The toning baths following the sulphur method were principally mixtures of gold terchloride and hypo. This latter substance was found to be a solvent of certain silver compounds by the Rev. J. B. Reade, in 1839, Mr. Talbot having previously fixed his prints with common salt. Prints, too, were fixed first in some cases, and toned afterward, washing away the free silver being more or less practiced in the mixed hypo. and gold and the sulphur toning processes. When fixing was employed before toning, it was usual to soak washed prints in a twenty per cent. solution of hypo. for a period of ten minutes, or until the soluble silver salts were removed, the resulting color being a disagreeable yellowish-brown. To improve the result so obtained the prints were passed into a solution of—
| Gold terchloride | 10 | grains. |
| Water | 20 | ounces. |
When toning action quickly followed, the yellow color giving place to that of a dark sepia tint. From this stage to that of mixing these two substances together was only a natural sequence, and effected a diminution of gold to the extent of one-fourth, as will be seen by the following recognized formula:
| Hypo. | 7 | ounces. |
| Water | 20 | " |
When dissolved, add—
| Gold terchloride | 5 | grains. |
| Dissolved in water | 20 | ounces. |
After mixing, a clear solution should result.
The sel d'or process followed, and was expected to give still better results. It was found, however, that the solutions would not keep; and as a considerable quantity of the gold salt was needed, it caused experimenters to search for a less expensive method. One decided point in its favor was the circumstance that prints suffered no loss of intensity during the operation, as they do in the case of all other toning methods. Briefly: the prints were well washed to extract free silver, and, after soaking five minutes in salt and water, they were passed into an alkaline solution composed of—
| Liquid ammonia | 60 | minims. |
| Water | 20 | ounces. |
Here they became very red. After washing in clean water the surface was flooded with a toning solution composed of
| Double hyposulphite of gold and sodium (sel d'or) | ½ | grain. |
| Hypo. | 1 | " |
| Water | 1 | ounce. |
Upon the print assuming a purple-gray color it was withdrawn and fixed in a sixteen per cent. solution of hypo. to dissolve the unacted upon silver chloride. Gold, when in a fine state of subdivision, is of a rich purple color. The layer obtained by deposition upon a silver image is very finely divided; hence the color. The only object in continuing the toning action beyond the stage at which a good surface color has been reached is to obtain a deposit of sufficient density to completely neutralize the red color of the organic silver image beneath; therefore, it is preferable, in forming a judgment of toning action, to examine proofs by transmitted light rather than by reflected only.
Before dealing with the various formulæ for alkaline toning I should like to step out of the golden track to say a few words on platinum tetracloride, PtCl4.
Experiment 3.—Platinum Toning.—The value of a platinum salt as a toning agent for silver images has been thoroughly demonstrated before you by Mr. Henderson, when he initiated us into the secrets of ceramic photography. My trials with this salt as a toning agent for paper proofs have only been partially successful. By that I mean that toning does take place when a dilute solution is employed, but the action is too tardy for demonstration here to-night, since anything like a black tone could not be obtained under half an hour. You will observe that the surface becomes covered with chloride, showing the necessity for copious washing. Yellow or discolored prints are bleached when toned in this bath, the whites becoming very pure. The formula here given is capable of producing a very good shade of brown in less time, and should be permanent, since platinum is a metal practically unaffected by the atmosphere; and I think there is good reason to suppose that if a thin coating of platinum could be deposited on the silver image, the protection offered would be more economical as well as stable. Something has already been done in this direction, but not in recent years.
The following is the composition we are now using:
| Platinum tetrachloride, sirupy solution, color of old East India sherry | 5 | minims. |
| Hydrochloric acid | 150 | " |
| Water | 20 | ounces. |
Wash away the free silver thoroughly, warm the toning solution to 70° Fahr., and fix in a twenty per cent. hypo. bath.
Mr. A. Watt, in the second volume of the News, gives a formula which runs as follows:
| Solution of platinum | 30 | minims. |
| Hypo. | 3 | grains. |
| Hydrochloric acid | 5 | minims. |
| Water | 5 | ounces. |
This bath is said to act instantly, but I have not had an opportunity to test it. The strength of the platinum solution here given is indefinite, but any of our experimental members can soon ascertain the amount of dilution necessary to obtain the most favorable results.
Alkaline Toning.—Owing to the bleaching action which occurs in toning silver prints with gold, which is slightly acid, certain experiments were made, and it was found that bleaching increased in proportion to the quantity of hydrochloric acid added. Now, in the action of toning chlorine is disengaged, and in order to render this powerful bleaching agent inert it has been proposed to introduce a substance capable of combining with it, and thus, in absorbing it, prevent undue loss of vigor. To obtain this a slightly alkaline toning bath became a necessity, and to Mr. Waterhouse we are indebted for the introduction of the alkaline salts (Hardwich).
Here is an example:
Experiment 4.
| Sodium carbonate (Na2HCO3) | 5 | grains. |
| Auric terchloride (AuCl3) | 1 | grain. |
| Water | 10 | ounces. |
Instead of the dry bicarbonate we will use a saturated solution. In this as well as the following experiments we shall tone three prints of the same subject, viz., ordinary, fumed, and preserved.
Mr. Maxwell Lyte has written on and investigated the properties of toning solutions a great deal more than most men, and we find the following emanating from Mr. Lyte:
| Sesquichloride of gold | 15 | grains. |
| Phosphate of soda | 300 | " |
| Distilled water | 1¾ | pints. |
And in the same communication it is mentioned that 180 grains of borax may be substituted for the phosphate with a like result. Therefore it will be seen that a borax toning bath is not of recent discovery, although it does not appear to have been quoted in many formulæ for at least a dozen years after its publication.
After the publication of Mr. Lyte's formulæ it was found that other salts behaved similarly; and among the first suggested we found sodium acetate, the qualities of which, extolled by the introducer, Mr. Hannaford, have since been verified by the whole photographic world. Here is one of the ordinary formulæ:
Experiment 5.
| Gold terchloride | 1 | grain. |
| Sodium acetate | 10 | " |
| "chloride | 10 | " |
| Hot water | 20 | ounces. |
Mix twenty-four hours before use. Neutralize with chalk or whitening (carbonate of lime).
The name of M. Le Gray must be mentioned as the originator of the lime and gold toning bath; although the original formulæ differ somewhat from the one now used, the results are identical. The original formulæ consisted first in washing away a portion of the free silver by soaking the proofs for a few minutes in two changes of water, then submitting them to the action of an auriferous bath, composed of
| Terchloride of gold, 1 per cent. solution | 1 | part. |
| Hyperchloride of lime (white powder) | 3 | " |
| Distilled water | 1,000 | " |
The action was complete in ten to fifteen minutes, when the prints required washing in two changes of water to free them from the chloride of lime remaining in the fibers previous to fixing in one to six of hypo. If the tone were satisfactory at the expiration of fifteen minutes, the ordinary washing could be proceeded with; if not, the proofs were submitted to a final bath composed of:
| Gold terchloride | 2 | parts. |
| Hypo. | 200 | " |
| Distilled water | 1,200 | " |
M. Le Gray says: "The proof ought not to be left in this bath less than fifteen minutes, as that is the minimum time necessary to insure the permanency of the picture; but it may be allowed to remain in it for as much longer as is requisite for obtaining the desired tone." Efficient washing in warm and cold waters completed the operation. Should any of our provincial members experience a difficulty in obtaining calcium chloride for their experiments, it can be easily made by causing dilute 7 to 3 hydrochloric acid to react on common whitening, and when neutral filter and set aside for the crystals to separate out.
Experiment 6.—The uranium and gold toning bath has many friends. The tones are said to be richer and to economize gold, while it is very easy to work. I am unable to give the author's name, but I can present a formula which has worked well in my hands. After washing away the free silver tone in the following mixture:
No. 1.
| One grain acid solution of gold terchloride | 1 | ounce. |
| Water | 7 | ounces. |
Neutralize with sufficient of a twenty per cent. solution of sodium carb. (Na2HCO3).
No. 2.
| Three grain solution of uranium nitrate | 1 | ounce. |
| Water | 7 | ounces. |
Neutralize as in No. 1. Warm each to 70° Fahr., and mix. The bath is then ready for use. It can be used repeatedly if desired by acidifying with citric acid and neutralizing before use; but nothing is gained by using it a second time.
There are methods of toning which resemble more or less those which have occupied our attention to-night; among them may be mentioned the tungstate bath, likewise citrate of soda. The vermilion bath, too, might afford sufficient matter alone for a lecturette. If some one experienced with it could be induced to bring it before us, I am sure it would prove interesting.
To coat plates perfectly, says H. S. Starnes in British Jour. of Photography, I found the following points were necessary:
1. That a certain quantity of emulsion should be flowed in one even stream all over the plate, instead of pouring the emulsion in a pool in the center of the plate and then dispersing it over the whole surface; because in the latter mode of coating large plates the gelatine is apt to commence setting before it is equally distributed, and an unequally coated plate is the result.
2. The plate ought to be put on the leveling-table before coating, and not be moved before the gelatine is set; because in the dull light of the dark room it is so difficult to prevent the emulsion running off the plate when putting it down on the leveling-table.
3. I found that if the emulsion be rubbed (so to speak) on to the glass there is much less chance of frilling, etc., than if it were poured on. I think it is because in the former case the gelatine is in firmer contact with the glass. When the gelatine is poured on to the plate the cold glass instantly chills it, and by the time the emulsion has reached the edges of the plate it has so far set as to have partially lost its power of adhesion to the smooth surface of the glass.
Two or three years ago, when it was the practice to warm the plates before coating, I found from a series of experiments I then made that when a plate was warmed before being coated the emulsion commenced setting on the surface of the film, and of course in setting contracted, thereby leaving a partial vacuum between the film and the glass. On development frilling was the consequence. I found, however, that, when pouring the same emulsion on cold glass, on the portion of the plate where it was poured on, the film instantly chilled and commenced to contract on to the glass, and it never frilled there; but toward the edges of the plate, as the emulsion had commenced to chill before they were covered, the film was not in such perfect contact with the glass. Any person can try the experiment by first coating a plate in the ordinary way, and on the second plate just pour a small pool of emulsion on the center; let both dry, and he will then see after exposure which frills the easier on development.
After a series of experiments I found that by brushing a substratum of emulsion on to the cold plate (with a brush made by binding a strip of wash-leather at the end of a strip of glass), and then pouring the full quantity of emulsion on to the substratum (for quarter-plates I used a small silver teaspoon, which held sufficient to cover that size of plate), I found I could coat plates far better and quicker and as easily as when coating with collodion, and I got over the difficulties of having frilling plates.
When only a few small plates are required—such as for experimental purposes—I believe this method is as quick and good as any; but when several dozen plates are wanted, any plan of coating them separately takes a long time. With my plate-coater I can coat a dozen plates in about the time I formerly took to coat one. When coating a number, I thought it would be best to lay them in rows on the leveling-shelves and draw the receptacle containing the emulsion over them, rather than keep the latter a fixture and run the plates under it either on an endless band or sliding shelves; because by the first mode the plates can be fixed close together, and the emulsion is less likely to get between them.
The coater is a species of wooden tray (of which the diagrams show the section), having a small slit in one of the bottom edges through which the emulsion passes in one even wave the whole width of the plate. The width of the coater is the same as that of the plate, though one six and a half inches wide can be used for either half or whole plates.
I find the best way of making it, so as to get the slit an equal opening the whole length of it, is to put the back, bottom, and two sides together first, as in Fig. 3. Then by putting a piece of very thin paper (A B) on the angle piece when the front piece of wood is put tight down on the paper and fixed in its place, and the paper is drawn out, it will be found that the slit is very even. In one coater I made I had the slit a little too wide an opening, and to reduce it I glued a piece of muslin over it. This I found was a great improvement, as it not only acted as a strainer, but it checked and caused a more even flow of the emulsion over the plate. I varnished the wood and muslin (except over the slit) with black Japan. 7458
To coat the plates I put them close together in rows on the leveling-shelf, as shown below:
A is a thin, narrow ledge of wood. B B B are thin pieces of wood, in the center of each of which is a small slot and thumb-screw. The plates are pressed against A by the pieces of wood, B, and the thumb-screws are then fastened. The plates are thus kept from slipping about. All this, of course, can be done in ordinary white light. The light is then made non-actinic; the melted emulsion is poured into the reservoir of the coater, which is put to the left hand edge of the outer row of plates. It is then lifted up on edge, as in Fig. 2, and drawn slowly over the row of plates, and so on until the whole of the rows are coated. Of course when not coating plates it is kept in a horizontal position, as in Fig 1. The emulsion on the plates is allowed to set without being disturbed; the shelf is then slipped into the drying-box until the plates are dry, so that they are not touched from the time they are coated until they are dry and ready for packing.
I am at present engaged in making a modification of this coater to hold a much larger quantity of emulsion at one time, when a large number of plates require to be coated. It is something the shape of a flat teapot.
Fig. 5.—A A is a piece of curved glass. B a piece of coarse ground flat glass, ground side uppermost, sliding in two grooves in the wooden side. C is the handle fixed to the wooden back.
A piece of thin paper is placed on the curved glass, and the ground glass pushed close up and fixed by two small wedges, D. The paper is then slipped out, leaving a narrow, even opening between the two glasses. The width of this opening can be varied by using thicker paper if the plates require to be coated with a thicker film. By using this form the coater can be more easily cleaned, as the ground glass can be slipped right out at the back, and probably in passing from the opening to the plates over the curved glass the wave of the emulsion will be equalized as well as when passing through the muslin.
In a recent paper in the Wochenblatt, says the Photographic News, this investigator relates his experience of gelatine emulsion containing chloride and iodide of silver. Gelatine films containing pure chloride of silver can only be used in the camera in exceptional cases; if, however, iodide be added, the resulting iodo-chloride films answer most of the purposes of a gelatino-bromide plate. It may be remarked that with gelatino-chloride emulsion an image is easily developed with pyro or oxalate; but unfortunately, fogging is very liable to set in. On strongly diluting the developing solution and adding a large proportion of bromide, it is possible to obtain a clear deposit, but the image is so thin that it is quite useless for practical purposes.
Gelatino-iodide films possess totally different properties. The development is extremely slow, without any tendency to fog; thus the addition of a restrainer should be avoided. Iodo-chloride emulsion can be prepared either by dissolving the chloride and iodide salts in the gelatine solution, and then adding by degrees the silver nitrate; or by making two separate emulsions of chloride and iodide of silver, and then mixing the two after the washing process. It should be noted that the properties of a compound or a mixture of the two haloids are very different. A negative of the spectrum impressed on an iodo-chloride film, prepared by mixing the two emulsions, shows two colored deposits. The red end of the spectrum as far as the G line is reproduced in the negative as a red tone, while that part of the spectrum from G extending to the violet appears as a grayish violet deposit. When using Stolze's potash developer, the difference of the two tones on the negative appears even more marked.
Experiments were instituted to determine the most suitable proportion of the silver haloids to be suspended in the emulsion. For this purpose three emulsions were prepared according to the following formulæ:
No. 1.—Iodo-chloride Emulsion.
| A.— | Ammonium chloride | 0.64 | gramme. |
| Potassium iodide | 0.05 | " | |
| Gelatine | 1.5 | " | |
| Water | 15 | c. c. | |
| B.— | Silver nitrate | 1.55 | gramme. |
| Water | 15 | c. c. |
No. 2.—Same as No. 1. but with 0.15 potassium iodide instead of 0.05; and 1.65 silver nitrate instead of 1.55.
No. 3.—Same as No. 1, but with 0.64 potassium iodide instead of 0.05; and 2.14 silver nitrate instead of 1.55.
To prepare the emulsion, A and B were heated in a water bath and then mixed slowly, with thorough shaking. The mixture, after an hour's cooking, was allowed to stand over night; the emulsion was next washed for seventy-two hours, and after slightly diluting, at once poured over the plates. The emulsions prepared according to formulæ 1 and 2 transmit blue light, which, however, is much brighter than that exhibited by gelatino-bromide emulsion. No. 3 emulsion transmits an orange light.
Previous to cooking the emulsion, a small quantity from each sample was spread on a glass plate, and, with the films prepared from the fully digested emulsion, were placed in sunlight. The unripe emulsion darkened much more quickly than that which had been digested. The colors of the exposed films prepared according to Nos. 1 and 2 were chocolate, and there was very little difference between the tones of the ripe and unripe emulsion. With the plates made by No. 3 formula there was, however, a great difference of color noticeable; thus, while the unripe emulsion yielded a deposit not unlike that of Nos. 1 and 2, the films prepared from the ripe emulsion assumed a grayish green color, which did not alter even after some weeks' exposure to daylight.
Messrs. A. Boake & Co., of Stratford, London, England, have devised a convenient apparatus for charging water with sulphurous acid which is useful in the making of photographic developers. The following description has been furnished by the firm:
The figure shows one of the siphons connected with a very convenient form of apparatus for preparing a solution of sulphurous acid in water, or of sulphites, as may be required.
The siphons are easy to manage, the flow of gaseous acid being regulated with the greatest nicety by simply turning the milled head shown in the engraving, the liquid acid being gradually converted into gas as the pressure is relieved. There is, moreover, no danger attending the use of this simple apparatus; sulphurous acid exerting at ordinary temperatures a pressure of about 30 pounds on the square inch, while each siphon is carefully tested under a pressure of 200 pounds on the square inch before being sent out.
In preparing a solution, say, of sulphurous acid in water, the ground stopper carrying the tubes for passing the gas should be removed from the glass jar in immediate connection with the siphon, and two-thirds filled with distilled water; the stopper is then to be replaced, and the second glass jar half filled with caustic soda solution. The soda solution is used to absorb any sulphurous acid not dissolved by the distilled water, and so prevent the escape of this irritating gas into the air. Solution of sulphite of soda, and also of bisulphite, can be prepared in a similar way, substituting only pure caustic soda solution for the distilled water employed in the case of preparing the solution of sulphurous acid; but we must rather devise the purchase of the pure solid forms of these salts specially prepared, and put up by us in one-pound stoppered bottles for use in photography; these preparations can be obtained either direct from the manufacturers or from any wholesale chemical firm. The siphons may be obtained either separately or already connected with the absorbing jars. It may be mentioned that these siphons contain about two and a half pounds of liquefied sulphurous acid, and can be refilled when required; but those requiring larger quantities can obtain the acid compound in copper drums.
The Photographic News says: It will be noticed that Messrs. Boake say there is no danger attending the use of the siphons, as the glass vessels are tested at a much greater pressure than that ordinarily exerted by the condensed sulphurous acid; but our readers must remember that a blow against a hard substance may cause the glass to become fractured, and that under these circumstances the bursting of a siphon might cause a serious injury. Still, if proper care is exercised, there need be no accident; but we would suggest that the condensed acid should always be kept in the coolest place available, as the pressure it exerts becomes much greater when the temperature is raised.
The above caution is necessary, as a bare statement that there is no danger may cause persons to handle the siphons without reasonable care. The risk is precisely analogous to that attending the use and handling of bottles containing ordinary aerated waters, only the irritating nature of the sulphurous acid must be taken into account. Instances have occurred in which serious injury has resulted from the bursting of a bottle of soda water; but few, if any, are deterred from the use of soda water or lemonade on this account.
The precipitation of tannin by a solution of gelatin is effected more completely and in a better condition for filtration if, besides ammonium chloride, as proposed by Schulze and Lehmann, there is also added a small quantity of chromium sulphate or of chrome-alum. The author proceeds in the same manner as Lehmann, but he adds to 100 c.c. of the solution containing sal-ammoniac from 5 to 8 drops of a solution containing 1 part chromium sulphate in 25 parts of water. In order to ascertain the end of the reaction, he filters small quantities into two test glasses of equal width, adds to the one a few drops of a solution of gelatin, observing if the two liquids, when held up against a sheet of black glazed paper, appear opaque or transparent. As long as a precipitate is formed, these portions and the washings of the little filters are poured back to the main quantity. If acetic or tartaric acid is present, the liquid should be neutralized before proceeding to the determination. Johanson points out that, though this method gives good results with the tannin of galls and of oak-bark, an extract of coffee gives no precipitate with solution of gelatin, so that caffeo-tannic acid cannot be determined in this manner. This shows that only quantities of tannin of one and the same kind can be compared with each other.
[Abstract of a paper read before the Chemistry Section of the British Association at Montreal.]
The author gave a resume of the work he had done in continuation of the researches of Bunsen, E. von Meyer, Horstmann, and other chemists, on the division of oxygen when exploded with excess of hydrogen and carbonic oxide. The following are the general conclusions arrived at:
1. No alteration per saltum occurs in the ratio of the products of combustion. The experiments made completely confirm Horstmann's conclusion; Bunsen's earlier experiments being vitiated by the presence of aqueous vapor in the eudiometer.
2. A dry mixture of carbonic oxide and oxygen does not explode when an electric spark is passed through it. The union of carbonic oxide is effected indirectly by steam. A mere trace of steam renders the admixture of carbonic oxide and oxygen explosive. The steam undergoes a series of alternate reductions and oxidations, acting as a "carrier of oxygen" to the carbonic oxide. With a very small quantity of steam the oxidation of carbonic oxide takes place slowly; as the quantity of steam is increased, the rapidity of explosion increases.
3. When a mixture of dry carbonic oxide and hydrogen is exploded with a quantity of oxygen insufficient for complete combustion, the ratio of the carbonic acid to the steam formed depends upon the shape of the vessel and the pressure under which the gases are fired. By continually increasing the initial pressure, a point is reached where no further increase in the pressure affects the products of the reaction. At and above this critical pressure the result was found to be independent of the length of the column of gases exploded. The larger the quantity of oxygen used, the lower the "critical pressure" was found to be.
4. When dry mixtures of carbonic oxide and hydrogen in varying proportions are exploded above their critical pressures with oxygen insufficient for complete combustion, an equilibrium is established between two opposite chemical changes represented by the equations:
(I.) CO + H2O = CO2 + H2.
(II.) CO2 + H2 = CO + H2O.
At the end of the reaction the product of the carbonic oxide and steam molecules is equal to the product of the carbonic acid and hydrogen molecules multiplied by a coefficient of affinity. This result agrees with Horstmann's conclusion. But Hortsmann considers that the coefficient varies with the relative mass of oxygen taken.
5. A small difference in the initial temperature at which the gases are fired makes a considerable difference in the products of the reaction. This difference is due to the condensation of steam by the sides of the vessel during the explosion, and its consequent removal from the sphere of action during the chemical change. When the gases are exploded at an initial temperature sufficiently high to prevent any condensation of steam during the progress of the reaction, the coefficient of affinity is found to be constant whatever the quantity of oxygen used—provided only the quantity of hydrogen is more than double the quantity of oxygen.
6. The presence of an inert gas, such as nitrogen, by diminishing the intensity of the reaction, favors the formation of carbonic acid in preference to steam. When the hydrogen taken is less than double the oxygen, the excess of oxygen cannot react with any of the three other gases present—carbonic oxide, carbonic acid, and steam—but has to wait until an equal volume of steam is reduced to hydrogen by the carbonic oxide. The excess of inert oxygen has the same effect as inert nitrogen in favoring the formation of carbonic acid. The variations in the coefficient of affinity found by Hortsmann with different quantities of oxygen are due partly to this cause, but chiefly to the varying amounts of steam condensed by the cold eudiometer during the reaction taking place in different experiments.
7. As a general result of these experiments it is shown that, when a mixture of dry carbonic oxide and hydrogen is 7459 exploded with oxygen insufficient for complete combustion, at a temperature at which no condensation of steam can take place during the reaction, and at a temperature greater than the critical pressure, an equilibrium between two opposite chemical changes is established, which is independent of the mass of oxygen taken, so long as this quantity is less than half the hydrogen. Within these limits the law of mass is completely verified for the gaseous system composed of carbonic oxide, carbonic acid, hydrogen, and steam at a high temperature.
At the July meeting of the Odontological Society of France, Dr. Cludius, from Grenoble, made the following demonstration of a new method of gold filling, saying:
We feel the necessity of making the operation of filling teeth with gold easier, if possible, especially in difficult cases, in order to lessen the fatigue of the operator, as well as to prevent the suffering of the patient, during hours without interruption, under the ceaseless blows of the mallet. The remedy has been sought in new forms of material, like sponge and crystal gold. These have not given any help in the performance of good operations, but have rather facilitated poor work. We are not in need of varieties in the forms of gold, but we ought to try and improve its manipulation, and this has recently been done in a novel manner by Dr. Herbst, whose rotation method has been mentioned in the dental journals within only a few months; and yet it seemed necessary that this great invention, made in Bremen, should take its way by America to come to us.
In the January meeting of the Odontological Society of New York, Dr. Bödecker mentioned it for the first time, describing the excellence of fillings made by Dr. Herbst in less than half the time that any mallet work would have required, and he expressed his intention of going to study the method with the inventor. Thinking that I was yet nearer to Bremen, I went thither, and found there Dr. W. D. Miller, who had come on the same errand. Mr. Brasseur had also written to Dr. Herbst, and it is by his (Mr. B.'s) invitation that I came to Paris to show you what I have learned in Bremen. To-morrow morning I shall show the method in the mouths of patients at the Dental College of France. Dr. Herbst did not patent his new method, to which may be given the name of "rotation gold filling." All he desires is that every one may try the system, and he feels himself already largely paid by the acknowledgments he is daily receiving.
He proves that by his way of rotation one is able to adapt the gold more perfectly to the walls of the cavity than by any other means hitherto employed. One can thus work gold in the very weakest teeth, because there is no force employed, yet the gold is as much condensed as by any mallet known.
The new instruments are very simple, and you may find them in the dental depots. One can easily prepare them for himself—at least the principal one, No. 5—by putting a broken burr in the hand-piece and holding it like a pen for writing until the rotating end of the burr is ground to a roof-like shape, on a dry Arkansas stone. Nos. 1, 2, 3, and 4 are smooth burnishers, and help to fix the first layers of gold in large fillings. They are afterward used as finishers, Nos. 9 to 17 are finishing burnishers, and No. 18 is a needle-point finisher.
The cavity is to be prepared in the usual way, but retaining points are very much less needed than for other methods. Take, for instance, a central cavity in a molar—and, moreover, the fundamental idea of this system is to transform all cavities to be filled into central cavities. Now fix several cylinders, of a size proportioned to the cavity, with a common plugger, and then take No. 2, or 3, or 4, and by a slow rotation polish the gold against the walls. If the gold does not stick directly, put in more cylinders with the plugger, and recommence the condensation with the burnisher. On this first layer of gold a second one is to be made to adhere; but the polished surface prevents, and here No. 5 finds employment in quick rotation and interrupted touches until the polish is gone. (I may here remark that the gold is condensed by this rotation and without pressure in a very remarkable way.) For large fillings, No. 5 is to have proportionate points,13 which, if too fine, will make holes in the gold, and the pressure is to be intermittent, in order to avoid the development of heat, which would be painful and irritating to the pulp.
All the instruments by use get gilded, and will not work longer without tearing out the gold; but this inconvenience may be prevented by occasionally rubbing them while in rotation upon a piece of tin.
The filling of the cavity is continued in the way above described.
Let us now take the case of two incisors with lateral cavities approximating one another. The two cavities, prepared as usual, are treated as if one, and the gold is at the same time introduced into both cavities, fixing some cylinders in the four corners by rotation of the proper burnishers, and condensation with No. 5, until they are filled, so that there appears to be a single mass of gold. No. 18 is then pushed with regular rotation between the teeth until the mass is quite separated, so that thin files, and disks, and tapes may be employed in finishing the fillings.
In filling similar cavities between the second bicuspid and first molar, after they are properly prepared, place a matrix and fill one cavity with shellac to retain the matrix, and distribute the resistance, and then fill the other like a central cavity, beginning at the cervical border, and pressing especially against the matrix at that point, work toward and finish at the middle of the crown. Having filled the first one, remove the shellac and fill the other in the same way.
The rotation and the pressure, if intermittent, do not produce heat—at least, not more than will render the gold cohesive.
Dr. Herbst filled for me two molars, carious to the cervical border, and very sensitive there, for which reason they had for years been filled with plastics, because I was afraid of perforation if retaining points were made, without which gold filling by malleting would not have been possible; and I was too nervous to sit three or four hours in the chair. Dr. Herbst filled both teeth by rotation, without retaining points, in a little more than one hour. Several gentlemen present have seen them and observed the severe tests to which Mr. Brasseur subjected them, and I may add that notwithstanding the great sensitiveness of the dentine and the proximity of the pulps, I felt not the least inconvenience from heat, and my own patients bear like testimony.
We will now split a crown filled in the hand, and you see that the gold is pressed into the smallest depressions of the interior surface, and is so uniformly condensed as to resemble an ingot, impossible to separate in pieces, yet you may note the different stages of the rotation.
I saw Dr. Herbst fill six cavities—some of them large ones—in front teeth, taking altogether at the same sitting about one hour.
It would be difficult to precisely describe the manipulation requisite for the great variety of cases presenting in practice, but I have explained to you in theory the typical ones in the hope of stimulating you to try this method of filling by rotation, which I look upon as one of the most ingenious modes yet given to our profession. The results are splendid, and the operator will thereby save much time and prevent great suffering on the part of the patient.
An important conference upon cholera was held in Berlin, at the Imperial Board of Health, on the evening of July 26. There were present Drs. Von Bergman, Coler, Eulenberg, B. Frankel, Gaffky, Hirsch, Koch, Leyden, S. Neumann, Pistor, Schubert, Skrezcka, Struck, Virchow, and Wollfhugel. The conference had been called at the instance of the Berlin Medical Society, whose President, Professor Virchow, explained that it was thought advisable Dr. Koch should in the first instance give a demonstration of his work before a smaller body than the whole Society, so that the proceedings might be fully reported in the medical press. He mentioned that Herr Director Lucanus and President Sydow had expressed their regret at being unable to be present, as well as many others, including Drs. Von Lauer, Von Frerichs, Mehlhausen, and Kersandt. Dr. Koch first showed various specimens of the bacilli and their method of preparation (see Berliner Klinische Wochenschrift, August 4). This resembles that for the tubercle bacillus, viz., drying on a cover glass and staining with fuchsin or methyl-olin. Koch then gave a history of his work while in Egypt and India. His post-mortem examinations led him to believe that the intestines were the nidus of the disease. At first his microscopical examinations were unsatisfactory, but finally he got fresh dejecta and acute cases, and then discovered the comma bacillus.
This, he said, is smaller than the tubercle bacillus, being only about half or at most two-thirds the size of the latter, but much more plump, thicker, and slightly curved. As a rule, the curve is no more than that of a comma (,), but sometimes it assumes a semicircular shape, and he has seen it forming a double curve like an S; these two variations from the normal being suggestive of the junction of two individual bacilli. In cultures there always appears a remarkably free development of comma-shaped bacilli.
These bacilli often grow out to form long threads, not in the manner of anthrax bacilli, nor with a simple undulating form, but assuming the shape of delicate long spirals—a corkscrew shape—reminding one very forcibly of the spirochæte of relapsing fever. Indeed, it would be difficult to distinguish the two if placed side by side. On account of this developmental change, he doubted if the cholera organism should be ranked with bacilli; it is rather a transitional form between the bacillus and the spirillum. Possibly it is true spirillum, portions of which appear in the comma shape, much as in other spirilla, e. g., spirilla undula, which do not always form complete spirals, but consist only of more or less curved rods. The comma bacilli thrive well in meat infusion, growing in it with great rapidity. By examining microscopically a drop of this broth culture the bacilli are seen in active movement, swarming at the margins of the drop, interspersed with the spiral threads, which are also apparently mobile. They grow also in other fluids, e. g., very abundantly in milk, without coagulating it or changing its appearance. Also in blood serum they grow very richly. Another good nutrient medium is gelatine, wherein the comma bacilli form colonies of a perfectly characteristic kind, different from those of any other form of bacteria. The colony when very young appears as a pale and small spot, not completely spherical as other bacterial colonies in gelatine are wont to be, but with a more or less irregular, protruding, or jagged contour. It also very soon takes on a somewhat granular appearance. As the colony increases the granular character becomes more marked, until it seems to be made up of highly refractile granules, like a mass of particles of glass. In its further growth the gelatine is liquefied in the vicinity of the colony, which at the same time sinks down deeper into the gelatine mass, and makes a small thread-like excavation in the gelatine, in the center of which the colony appears as a small white point. This again is peculiar; it is never seen, at least so marked, with any other bacterium. And a similar appearance is produced when gelatine is inoculated with a pure culture of this bacillus, the gelatine liquefying at the seat of inoculation, and the small colony continually enlarging; but above it there occurs the excavated spot, like a bubble of air floating over the bacillary colony. It gives the impression that the bacillus growth not only liquefies the gelatine, but causes a rapid evaporation of the fluid so formed. Many bacteria also have the power of so liquefying gelatine with which they are inoculated, but never do they produce such an excavation with the bladder like cavity on the surface. Another peculiarity was the slowness with which the gelatine liquefied, and the narrow limits of this liquefaction in the case of a gelatine disk. Cultures of the comma bacillus were also made in agar-agar jelly, which is not liquefied by them. On potato these bacilli grow like those of glanders, forming a grayish-brown layer on the surface. The comma bacilli thrive best at temperatures between 30° and 40° C., but they are not very sensitive to low temperatures, their growth not being prevented until 17° or 16° C. is reached. In this respect they agree with anthrax bacilli. Koch made an experiment to ascertain whether a very low temperature not merely checked development, but killed them, and subjected the comma bacilli to a temperature of -10° C. They were then completely frozen, but yet retained vitality, growing in gelatine afterward. Other experiments, by excluding air from the gelatine cultures, or placing them under an exhausted bell-jar, or in an atmosphere of carbonic acid, went to prove that they required air and oxygen for their growth; but the deprivation did not kill them, since on removing them from these conditions they again began to grow. The growth of these bacilli is exceptionally rapid, quickly attaining its height, and after a brief stationary period as quickly terminating. The dying bacilli lose their shape, sometimes appearing shriveled, sometimes swollen, and then staining very slightly or not at all. The special features of their vegetation are best seen when substances which also contain other forms of bacteria are taken, e. g., the intestinal contents or choleraic evacuations mixed with moistened earth or linen and kept damp.
A most important statement was that the comma bacillus seems to be killed by the bacteria of putrefaction, and consequently agents that destroy the latter organisms without the former may really do injury, by removing from the cholera bacillus an impediment to its growth.
As for destructive agents to the bacillus, he found it killed by solutions in the following proportions: oil of peppermint, 1 in 2,000; sulphate of copper, 1 in 2,500 (a remedy much employed, but how much would really be needed merely to hinder the growth of the bacilli in the intestine!); quinine, 1 in 5,000; and sublimate, 1 in 100,000.
In contrast with the foregoing measure for preventing the growth of these bacilli is the striking fact that they are readily killed by drying. This fact is proved by merely drying a small drop of material containing the bacilli on a cover glass, and then placing this over some of the fluid on a glass slide. With anthrax bacilli vitality is retained for nearly a week; whereas, the comma bacillus appears to be killed in a very short time.
Dr. Koch having found and cultivated the comma bacillus and ascertained its distinctive character, next proceeded to investigate its relation to cholera. In all there were now about one hundred cases of cholera in which the bacillus had been found, while it was never found in connection with other diseases. Three different views, said the speaker, as to its relation to the cholera process are tenable:
1. That the disease favors the growth of these bacilli by affording them a suitable soil. If so, it would mean that the bacillus in question is most widely diffused, since it has been found in such different regions as Egypt, India, and France; whereas the contrary is the case, for the bacilli do not occur in other diseases, nor in the healthy, nor apart from human beings in localities most favorable to bacterial life. They only appear with the cholera.
2. It might be said that cholera produces conditions leading to a change in form and properties of the numerous intestinal bacteria, a pure hypothesis; the only instance of such a conversion refers to a change of physiological and pathogenic action, and not of form. Anthrax bacilli under certain conditions lose their pathogenic power, but undergo no change in shape; and that is an instance of a loss of pathogenic properties, while there is no analogy to support the view of the harmless intestinal bacteria becoming the deadly cholera bacilli. The more bacterial morphology is studied, the more certain it is that bacteria are constant in their form; moreover, the comma bacillus retains its special characters unchanged through many generations of culture.
3. Lastly, there is the view that the cholera process and the comma bacilli are intimately related, and there is no other conceivable relation but that the bacilli precede the disease and excite it. "For my own part," said Dr. Koch, "the matter is proved that the comma bacilli are the cause of cholera."
Dr. Koch then described his attempts to inoculate lower animals with the bacillus, and explained the cause of his failure in the natural immunity of the animals against the disease.
In advocating the local Indian origin of the disease he said: That the virus can be reproduced and multiplied outside the body is apparent, since the bacillus can be cultivated artificially, and its growth is not affected by comparatively low temperatures. Probably it does not grow in streams and rivers, where, owing to the current, a sufficient concentration of nutrient substance does not occur; but in stagnant water and at the mouths of drains, etc., vegetable and animal refuse may accumulate and afford the necessary nutriment. Thus is explained the propagation of cholera by the subsoil water, and the increase of epidemics with the sinking of its level, which lessens the flow and diminishes the amount of surface water. Admitting the dependence of cholera upon this micro-organism it is impossible to conceive the disease having an autochthonous origin in any particular place; for a bacillus must obey the laws of vegetable life, and have an antecedent; and since the comma bacillus does not belong to the widely diffused micro-organisms, it must have a limited habitat. Therefore, the occurrence of cholera on the delta of the Nile does not depend on its resemblance to the delta of the Ganges; but the disease must have been imported there as it is into Europe. It was once thought that an outbreak in Poland had a local origin until it was discovered to have been introduced from Russia. Again, about ten years ago, there was a sudden outbreak at Hamar (Syria), thought to be an instance of local origin, but erroneously, as shown by a statement of Lortet, who told Koch, when at Lyons, that the epidemic had been introduced into Hamar, where he was at the time, by Turkish soldiers from Djeddah. All great epidemics of cholera began in South Bengal, where the conditions for the development and growth of the bacillus are most perfect.—Med. Record.
The notes of a few cases of the use of the hydrochlorate of cocaine will illustrate its perfect efficiency in some and its apparent inertness in others, and may help toward its proper application and general appreciation.
In a double extraction of hard cataract there was no pain produced by the graspings of the conjunctiva in the fixation of the eyes, in the corneal incisions, and in the iridectomies.
A 4 per centum solution was freely brushed over the entire conjunctival surface three times, at intervals of ten minutes, and the operations were commenced in forty minutes after the first application. No irritation was produced, and the only sensation described was that of "numbness and hardness." The entire conjunctival surface seemed insensible to repeated pinching with the fixation forceps.
In a single extraction of hard cataract a 4 per centum solution was brushed over the ocular and palpebral conjunctiva, with the eyelids freely everted. Three applications 7460 were made at intervals of ten minutes, and the operation was performed at the lapse of twenty-five minutes. The patient asserted decidedly that she felt no pain whatever.
Preparatory to the operation for uterine procidentia and rectocele, the vaginal and labial mucous surface was wiped dry, and a 4 per centum solution of the hydrochlorate of cocaine was thoroughly brushed over it. The sensitiveness was tested at three intervals of ten minutes each, and the application was repeated three times. There appeared to be at no time any decided loss of painful sensibility, and the operation was finally performed under the anæsthesia of sulphuric ether.
For the removal of a rather large tarsal tumor, the ocular and palpebral conjunctiva and the exterior of the eyelids were brushed with the solution as previously used, at intervals of ten minutes, and the excision was performed at the lapse of forty minutes. The operation seemed to be as painful to the patient as if performed without an attempt at anæsthesia.
For the operation for lachrymal obstruction the application was made in the same manner and at the same intervals. The slitting of the punctum and caniculus gave no pain, but the passage of the dilating probe down the lachrymal canal seemed to produce some uneasiness.
Prior to applying nitric acid as a caustic to a syphilitic ulcer on the tongue, the same manner and number of applications were repeated, the tongue having been wiped dry and held protruding between the teeth. No pain was produced on the thoroughly benumbed tongue.—Med. News.
The outcome of several public inquiries which have taken place during the last year or two, and of much valuable data derivable from other sources, establishes, we think, a well marked advance with reference to sewage disposal; and it may be of use, as well as of interest, if we lay before this Congress the conclusions which, we conceive, are deducible therefrom. We propose to deal with the subject under the following heads: 1. Sewage disposal on land. 2. Sewage disposal by chemical treatment. 3. Sewage disposal by discharge into a tidal river, or into the sea, without treatment.
The object of dealing with sewage on land may be taken as twofold, namely, to purify it (which is the sanitary object), and to utilize its manurial products (which is the agricultural object). Where want of skill or where prejudice has existed, these two have not been properly separated, and the results have been in many cases unfavorable to sewage disposal on land from either of the before mentioned points of view. It has been regarded as an axiom that clay land cannot be employed to clarify sewage. This is true when it is proposed to pour the sewage on it as if the land were porous. Very recent experience, however, has led to clay land being converted from an impervious to a pervious condition, by which it has been successfully utilized. This is effected by digging out the clay to a depth of about 6 ft., burning it into ballast and replacing it in layers interposed with an occasional layer of open alluvial soil, the whole area being well drained with a free outlet for the effluent. We have successfully carried this plan out, and with this result, that whereas it was not possible previously to clarify the sewage of 100 people to an acre of clay land, the prepared filtration area has been able to continuously clarify the sewage of about 1,500 people to the acre. The cost of converting clay land into this form of filter may be taken as varying from £750 per acre to £1,000 per acre, according to local circumstances. One area which we have just completed has cost £1,000 per acre. Before sewage is passed on to these filters (or on to land) it should be strained so as to remove the larger particles. The best arrangement for this purpose is to pass the sewage upward through a straining medium (not downward), and to run the solids from the bottom of the straining tank on to a low lying piece of land for digging in as they are run out. Where such a filtration area is made to form part of a sewage farm it acts as a safety valve, and enables the land and crops to have a rest when they do not require further irrigation; at the same time the process of purification is not interrupted. If open, porous land is available for sewage purification, and if it can be drained 6 ft. deep to a good free subsoil, so that the effluent can get readily away, we find that the sewage of from 600 to 700 people can be dealt with on each acre per annum with both good agricultural and sanitary results.
In our address as President of the Engineering and Architectural Section of the Congress of this Institute at Newcastle upon Tyne, in 1882, we directed attention to the important investigation which had been conducted by Mr. R. Warrington, of Rothamsted, the result of which was to show the action which goes on in the soil when sewage is passed through it. Further information which the same observer has published since that date is of equal value, and deserves to be read by all who have to advise in regard to sewage disposal on land. The process of "nitrification" (as it is termed), which he has so fully investigated, consists in the conversion into nitrates (which serve to nourish plant life) of the organic matter in sewage. This takes place by the action of a living ferment of the bacteria family, which is created by and feeds on the impurities in sewage, and these organisms both consume the impurities and convert them into nitrates. The action of living agents thus brings about the oxidation of the organic matter in sewage, just as worms, larvæ, fungi, and insects feed on the vegetable matter in the soil, increasing the amount of nitrogenous material in it. Experience during the past year or two has proved the feasibility of preserving green crops in a succulent state by compressing them in silos, so that they can be utilized for cattle fodder in the winter. This system deserves notice in connection with sewage farming, as we are of opinion that it will prove a valuable means of getting over the well known practical difficulty which is experienced of finding a market for the large amount of green crop which is produced by sewage irrigation. In speaking of this system the term "silo" is applied to the artificial chamber or receptacle for green crops (such as grass, vetches, clover, etc.).
The term "silage" is applied to the crop thus treated, and the term "ensilage" is applied to the process of making "silage." The details of the construction of silos cannot be referred to here, beyond stating that what is required is to construct a pit or chamber either in the form of an excavation in the ground, with a brick or other lining, or by building it above the ground. The object is to enable the green crop to be deposited in an air and water tight chamber, in which pressure can be applied to the crop to compress it. This is effected in some cases by well treading the crop after it is laid in the silo, and then spreading layers of earth to about a couple of feet, and pressing the covering well down. Another way is to construct the silo with a movable covering of the exact size and shape of its interior. This cover is raised and lowered by suitable chains and rollers. After the crop is placed in the silo, the cover is lowered and weighted so that a thorough compressing is effected; the weight applied giving about 200 lb. or so per square foot of surface. Salt is sometimes added as the crop is placed in the silo. A crop thus dealt with is stored for months; when the silo is opened the fodder is found preserved, and in a state readily taken to by cattle. It is desirable to choose the site for the silos so that the fodder is preserved somewhere near the place of consumption; also to lay out the works so that as little handling as possible is required. For instance, the silo should be on sidelong ground, so that the crop can be carted and tipped at a high level, and the silage taken out for use at a lower level.
In the last edition of our book on "Sewage Disposal," in speaking of precipitation we said that "the purification of sewage by chemicals has been the subject of misapprehension, owing to the extravagant advantages which have been claimed for the system by its advocates." This is even more true now than it was two years ago, inasmuch as in the recent scheme for dealing with the sewage of the Thames Valley chemical treatment per se was relied on to produce from the sewage of a future population of 350,000 an effluent at all times fit to be discharged at one point into the river Thames above London; but the Parliamentary Committee rejected it. One part of the report of this Committee deserves attention, when speaking of sewage treatment by chemicals. It is as follows: "Your committee believe that in these cases the process of filtering the chemically purified effluent through earth ought, if possible, to be adopted, which was not provided for in the scheme under their consideration." This opinion is exactly in accordance with our experience, and is that which we have held throughout. It is at the root of the whole matter, because efforts are made by those interested in chemical processes to attain as high a standard of purity as possible with the attendant heavy expense of chemicals. Experience shows that it is impossible at all times and seasons to be sure of a constant and uniformly high standard of purity, and that chemical works should be supplemented by a filtration area, however small. The addition of this, however, enables a lower standard of effluent from the precipitation tanks to be admissible, and this can be attained with very simple and inexpensive chemicals.
In the course of our practice we have had to advise as to the majority of the processes, and to design the works for their being carried into operation. We have found that the cost of such works complete varies from 0.091 to 0.166 pound per head of the population, and that the average cost of the works at several towns which we have been connected with is 0.123 pound per head. This figure may be conveniently followed by that of the cost of treatment, which we find varies from 0.036 to 0.110 pound per head per annum, and an average of several places gives 0.06 pound per head per annum. The above figures apply only to places where the very highest standard was sought to be attained, but our more recent experience leads us to modify the arrangement of the works and the cost of treatment, so as to rely on filtration of the effluent as an important factor. We estimate that under these conditions the cost of the works complete would be about 0.075 pound per head, and the cost of treatment 0.04 pound per head per annum. The disposal of the sludge has always been a difficulty in these works, but this is now overcome in two ways: either by digging it into the ground, as is done at Birmingham now, or by pressing it into cakes in filter presses. It is found at Birmingham that one ton of sludge with 90 per cent. of moisture is produced from 1,000 people. There the lime process is used. We have found that about one ton to 2,000 people is produced where a salt of alumina or iron is used with the lime. At Birmingham the sludge is dug into the land adjoining the works, and it is found that one square yard of land will take one ton of sludge with 90 per cent. of moisture once in three years, which results in three yards of land being required to be provided for each ton of sludge. This system of digging in sludge is successfully carried out as regards freedom from nuisance. Where land is not available to dig in the sludge, it is necessary to make it portable for removal and disposal away from where it is produced. This is best effected by filter presses. Appliances are made for this purpose, by which the sludge is pressed to a consistency of about 50 per cent. of moisture. The cost of effecting this is about 0.007 pound per head per annum. It is found in practice that where the sludge is produced by straining the solids from sewage before passing it on to land for purification, it requires a little lime to enable the press to work well. About two barrow loads of lime for each ton of pressed sludge suffices.
It has been thought that the cost of precipitation would be covered, and even a profit gained, by the sale of the sludge. This hope, however, is not nearer realization now than it was in the time, now gone past, when chemical processes were relied on to turn sewage from a profitless into a profitable commodity. There is, consequently, less justification now than there was at that time for adopting a precipitation system for sewage disposal. It is entirely a question of carefully considering the engineering and financial points involved, regardless of the sanguine representations of interested or enthusiastic advocates of any particular system. As the estimated manurial value of the sludge which is precipitated from sewage by the addition of chemicals does not seem to be capable of realization, we think that probably the reason may be found in the fact that the chemicals arrest that process of decomposition which is essential to the conversion of the organic matters into nitrates for vegetation to utilize.
This explanation will be understood in the light of what we have already described in regard to "nitrification." If this view is correct, it would follow that the more completely and permanently the sludge is deodorized by the chemicals, the less capable is it of passing through the necessary stages of decomposition by which its manurial value can be realized. As mistakes are constantly being made in regard to the weights of sludge with varying degrees of moisture, the following table may be useful:
| Tons. | Per cent. | Tons. | Per cent. | |||
|---|---|---|---|---|---|---|
| 100 | of sludge with 90 of moisture = | 50 | with | 80 | ||
| 100 | " | " | " | 33.3 | " | 70 |
| 100 | " | " | " | 25 | " | 60 |
| 100 | " | " | " | 20 | " | 50 |
| 100 | " | " | " | 16.6 | " | 40 |
| 100 | " | " | " | 14.3 | " | 30 |
| 100 | " | " | " | 12.5 | " | 20 |
| 100 | " | " | " | 11.76 | " | 15 |
We will next deal with the conditions which should be fulfilled where it is sought to utilize a river or the sea into which to cast the sewage of a town. If it can be ascertained beyond question that at the proposed point of discharge the currents at all times will carry the sewage right away, and will not at the same time produce mischief at a distance (which is often omitted from the consideration), then that arrangement may be accepted as a good one. This, however, seldom occurs.
A river has been looked upon by manufacturers and local authorities as the natural carrier of their refuse from their district. This view has been persevered in, in spite of the River Pollution Prevention Act of 1876, which is practically a dead letter. The public, however, who use a river either for pleasure purposes or for obtaining their water supply, have of late years grown more and more united in their efforts to stop this abuse; and there is no doubt that these efforts will eventually succeed. In a paper which we read last year at the Congress at Glasgow, we pointed out the steps that were necessary to be taken to render this act operative, and we refer our hearers to that paper if they wish to follow the matter further. The effect of discharging sewage matter into a river has been the subject of much controversy among chemists. Some allege most positively that the injurious properties in the sewage are indestructible. This has led to alarmists demanding that under no circumstances ought sewage to pass untreated into a river.
We have given considerable attention to this vexed question, as it requires to be grasped by any engineer who has to advise on the selection of sewer outfalls, and it appears to us that the balance of evidence is against the alarmists. Every river has a certain power of oxidizing impurities in proportion to the extent of oxidation of the river itself. Besides this, there are the powerful purifying influences exercised by the plants and animalcules which exist in rivers.
It has been ascertained that entomostraca consume dead animal matter; and where this is wanting they do not live, but where it is in abundance they thrive. It follows, then, these minute animals exercise an important function in absorbing sewage impurities. They multiply prodigiously in these impurities, and are both created by them and fed upon them, converting foul and dangerous matters into harmless ones, in a similar way to that which we have referred to as nitrification when speaking of the action of bacteria in the soil. Considering that these organisms arise from and are fed on concentrated filth, it is obvious that they cannot live when the conditions favorable to their existence disappear. This would be the case when the sewage is discharged into a large volume of water with a different temperature to that which suits them, and with powerful oxidizing influences at work. These conditions, added to the difficulty they must experience to find their natural food—namely, concentrated sewage—where the sewage matter becomes so greatly diluted, accounts for the fact that in a short run of a good river sewage impurities largely disappear. The action of weeds and plants also aids purification to a very large extent. Minute plants, such as confervoid algæ and the like, also assist in oxygenating the river, as when exposed to light they decompose carbonic acid, and liberate oxygen.
The practical question which has to be answered in every case where sewage is proposed to be discharged into a river requires to be approached from two points. The first is whether a nuisance will be caused at the spot to which objection would be taken. If this is likely to be the case, then the fact that the sewage will get purified in a short run of the river does not meet the objection. The second point requires a careful consideration of the condition of the river, both from an engineering as well as from a chemical and biological point of view. Decisions on these matters have too often been arrived at in a rough and ready way. They require skillful treatment, as the interests—both commercial and hygienic—which are affected are too great to permit of them being dealt with by any who are not well informed and careful. The general conclusions which we deduce from our observations are as follows:
1. That chemical precipitation is not so necessary now as it was considered to be a few years ago, in cases where land for irrigation is not procurable.
2. That the efforts to profitably remove the manurial elements from sewage by chemicals not having been successful, the system should be adopted per se only where a filtration area cannot be obtained.
3. That the success which has attended the construction of filtration areas where the land is clayey, and the successful results which have been obtained from a combined straining of sewage and of subsequent filtration through small areas of artificial filters, point to the adoption of one or other of these systems in many cases where chemical treatment would previously have been advised.
4. That the injurious effects of passing untreated sewage into a river depend upon not merely the relative volumes of the sewage and the river, but chiefly upon the power of the river to oxidize the sewage, which power is in proportion to the extent of oxidation of the river itself.
An article in the local news columns of the Tribune says:
The loud outcry made a few years ago against the old fashioned plush covered spring cushions, then used in street car for seats and backs, caused them to be removed and set car builders at work to make a car that would be light, healthy, and comfortable. The general plan of perforated wooden seats with plain backs has been adopted by all the companies. They are covered with a fine quality of heavy Axminster carpet during the winter, and in the summer nearly all the cars have only wooden seats and backs. Open cars are used on a number of routes during the summer, and this is conducive to the health of passengers. The only particular difference in the furniture of the cars is the mats used on the floor. Seven of the lines use sectional wooden mats of plain or ornamental design, while three retain cocoa mats. Wooden mats are the easiest to clean. Cocoa mats retain moisture on damp or rainy days, and emit a musty odor. There are four sets for each car, and they are changed every trip on rainy days.
The First and Second Avenue routes, under one management run 150 cars; the Third Avenue, 180; the Fourth, 75; the Sixth, 88; the Broadway and Seventh, 135; the Eighth and Ninth, 160; and the Tenth, 120. At the stables of each the same general arrangement for cleaning cars is used, while the details only are different, being regulated by the judgment and experience of superintendents. From six to fifteen men are employed for cleaning cars by the different companies.
After every round trip that a car makes, it is taken to the stable, the mats are taken off the floor, and two men with 7461 brooms and specially constructed brushes give it a thorough sweeping and brushing. After a car makes its last trip at night, it is run upon what is termed the washstand, which is a large turn table surrounded by hydrants. Everything movable is taken out of it, and water is played from a hose on the inside and outside, while four men with scrubbing brushes and stiff brooms remove whatever dirt has accumulated during the day. After this operation the car is run upon a side track, and two men dry the inside and polish the windows.
While passengers find fault with the untidiness of street cars, superintendents have a word also of complaint against passengers. If men would not convert a car into a spittoon for the reception of cigar stumps, tobacco spit, and quids, and a garbage box for nut-shells, fruit rinds, cores, and pits, the remnants of lunches and old papers, it would be much easier to keep up a cleanly appearance. Section 167 of the Sanitary Code, which provides that no soiled article of clothing or bedding shall be carried on street cars, except on the front platform, is strictly enforced by all the companies, and it is worth a conductor's position if he is proved derelict in this respect.
Nearly all the car companies build their own cars, and all have repair shops at their stables, and as soon as a car is damaged by a collision it is sent at once to the shop and repaired. Men are detailed to keep a strict watch over all the working parts of cars.
No metal or plate has yet been found of which to make a hand railing that will keep bright and untarnished. Many experiments have been tried, but the hardest plate that can be obtained will not stand the friction of the hands longer than two months, before the plated metal will show through. Cars are painted and varnished at least once a year. The various parts of the car last different periods. The wheels average about eighteen months on long routes; on short routes, about two years. Steps and platforms last about five years. There is no particular limit for the floors and framework, as they are but little worn. Cars are frequently built up from an old floor or framework, but at the end of about fifteen years there is but little left of the original car.
When, by means of a tube of from 2 to 5 millimeters in diameter, we gently blow tobacco smoke against a wet pane of glass, we produce very fugitive rings. If we operate with a closed vessel the rings are fixed, the current being itself uniform. But the experiment that shows the phenomenon perfectly is the one that consists in rendering the current automatic by means of an aspirator—an arrangement analogous to that devised by Mr. Nickles for analyzing the flame of a candle. A tapering glass tube or, better, a metallic blow pipe traverses a cork which hermetically closes a large bottle having a cock beneath and filled with water (Fig. 1). The nozzle of the blow pipe entering the center of the flame, and the cock being open, the liquid flows, and a column of white smoke descends vertically to the surface of the water, where it forms several concentric rings whose relief soon increases with the thickness of the heavy smoke, which finds no exit. These rings have a diameter so much the greater in proportion as the current is stronger (Fig. 2).
Unfortunately, the number of the rings soon diminishes in measure as the stratum of smoke that remains upon the surface of the water becomes thicker. Finally, there remains but a single ring, which has a thickness in the center of more than 0.015 m. (Fig. 3).
Instead of the smoke of a candle, we may employ that of a cigar or of a tobacco pipe. We thus avoid a deposit of fatty matter, which, in the first case, soon clogs up the tube, if it is too fine a one, and thus puts a stop to the experiment.
Several circumstances are known under which rings or crowns are produced. (1) For example, in the spontaneous combustion of phosphureted hydrogen, the resulting white vapors of phosphuric acid rise, and roll round in horizontal white crowns when the air is calm (Fig. 4). These crowns, whose diameter keeps on increasing, end by separating into strips that dissolve in the humidity of the air. (2) The crowns that we sometimes observe in calm weather around cannons at the moment of firing have the same origin, although they are of a different nature, and spread horizontally to a certain distance. With vertical howitzers the crowns are horizontal, and very beautiful when seen from beneath, since they rise vertically. (3) As well known, a cardboard box having two apertures in the center of two opposite sides, when filled with smoke and struck upon one of these sides, allows the escape through the opposite aperture of curling rings of smoke. (4) Steam escaping into the open air, through the intermittence of a vertical eduction pipe, sometimes makes its exit in the form of circular or elliptical crowns.—La Nature.
The hyacinth is a native of the East. When it was introduced into England, in 1596, only four varieties of it were known, but the Dutch gardeners soon made wonderful progress in its culture, and, along toward the end of the sixteenth century, had produced at least two thousand varieties.
This plant is well adapted for house decoration in winter, when flowers are rare. Its culture requires but little care. When the bulbs have taken root in a dark place they are gradually brought into the light, and placed where the temperature is moderate.
Is a regular changing of the water favorable to the development of this plant? Many florists doubt it, and it is often recommended not to change the water, but only to replace that which has been lost through evaporation. Others are of a contrary opinion, and assert that the less favorable results that are obtained when the water is changed are merely due the fact that the roots are injured when the plant is taken out of the glass.
With the old style of glasses it has always been difficult to renew the water regularly and keep the glass clean, but this inconvenience has disappeared in the glasses invented by Mr. J. C. Schmidt, of Erfurth.
Fig. 1 represents one of these glasses, and Fig. 2 shows the details. As may be seen, the tube, a, which contains the bulb, may be removed from the glass, b, without the plant being touched or its roots disturbed. The glass, b, may thus be easily cleaned and filled with fresh water as often as necessary.—Science et Nature.
The meeting of the American Association last year at Minneapolis attracted a larger attendance of botanists than usual. Without much consultation, a meeting of those interested in botany was called, a president and a secretary were chosen, and discussions, short communications, and papers upon botanical subjects listened to. The Botanical Club was thus inaugurated; and before the close of the session it was decided to do what was possible to secure a larger attendance of botanists at the next gathering in Philadelphia.
Although during the interim the prospect of a good attendance at the Philadelphia meeting had been fair, the most sanguine were surprised to find that, as early as Monday preceding the opening, a number of botanists had arrived in the city; and by the following day a larger gathering could have been assembled than the total attendance at Minneapolis.
The first meeting of the club, of which several were held between Friday and Wednesday, was responded to by an attendance of about thirty—a little below the average attendance for the subsequent meetings. Prof. W. J. Beal, of Lansing, Mich., the president, took the chair; and Prof. J. C. Arthur, of Geneva, N. Y., was appointed secretary to fill the vacancy caused by the absence of Professor Coulter. A paper by Dr. N. L. Britton, of New York, on the composition and distribution of the flora of New Jersey, was read. The surface-features of the State were given, and the corresponding vegetation described. The work of cataloguing the plants is being done under the supervision of the State geological survey. The list at present has reached the very large total of nearly fifty-five hundred.
Prof. C. R. Barnes, of La Fayette, Ind., spoke of the course of the fibro-vascular bundles in the leaf-branches of Pinus sylvestris. The two needle-leaves at the end of each short lateral axis contain each a paired bundle. The question at issue was whether this structure represented one or a pair of bundles, or whether it might not be a segment of the fibro-vascular ring of the stem. A study of the early stages shows that the first change in the stem is to divide the fibro-vascular ring into halves at right angles to the plane of the leaves; and subsequently these divide again, sending one branch of each to each leaf. The paper led to much discussion by Professors Buckhout, Macloskie, and others.
Dr. Bessey, of Ames, Ia., described the opening of the flowers of Desmodium sessilifolium. They expand partially in the usual manner, then remain stationary till a particular sensitive spot at the base of the vexillum is touched by an insect, when the wings and keel descend with a jerk, the stamens are released, and the insect dusted with pollen.
Professor Mackloskie, of Princeton, N. J., described the method of cross-fertilization of Geranium maculatum by bumblebees. Professor Dudley, of Ithaca, N. Y., spoke of the torsion of stems of Eleocharis rostellata, and also on the protogynous character of some species of Myriophyllum. Mr. William H. Seaman, of Washington, D. C., advocated the use of rather thick oblique sections in studying the structure of the fibro-vascular bundle—a method that called forth a very strong protest.
Professor W. J. Beal gave a paper concerning the manner in which certain seeds bury themselves beneath the soil, which was discussed by Professors Bessey, Rothrock, and others. A paper by Prof. W. R. Lazenby, of Columbus, O., on the prolificacy of certain weedy plants, embraced careful estimates of the average number of seeds produced by individual plants among various kinds of weeds. Dr. J. T. Rothrock, of Philadelphia, addressed the club on some phases of microscopic work, alluding particularly to microscopic work, alluding particularly to micro-photography, its importance to the investigator, and the ease of execution.
Dr. Asa Gray called attention to the interesting discovery of Mr. Meehan regarding the mode of exposing the pollen in the common sunflower. He had found that, contrary to the teachings of the text books, the pistil and stamens develop together until reaching full length, when the filaments rapidly shorten, and the anther tube is retracted, exposing the style covered with pollen, the further changes being the same as usually stated. This Mr. Meehan construed to be a device for self-fertilization; while Dr. Gray showed that, although bees carried pollen from one flower to another of the same head, they also carried it from head to head, which constituted crossing in the fullest sense. An interesting discussion followed, in which Professor Beal suggested that an excellent experiment would be to cover up the heads and ascertain if any fertile seeds were produced. Dr. Gray thought it very likely there would; for, when cross-fertilization is not effected, self-fertilization often takes place. Mrs. Wolcott had proved this to be so; for, in covering up the flowers to keep birds away, she found that plenty of seeds were formed.
Dr. George Vasey, of Washington, gave some notes on the vegetation of the arid plains, which was followed by observations on the curvature of stems of conifers by Dr. Bessey, in which he noted the bending of stems one, two, and even three years old.
Mr. Thomas Meehan discussed the relationship of Helianthus annuns and H. lenticularis; showing that there was a constant difference in the form of the corollas, the former being campanulate, and the latter tubular. The two are treated as one species in Gray's Synoptic Flora of North America; the one being considered a cultivated form of the other, a view from which the speaker dissented. Mr. Meehan then spoke upon the fertilization of composites; concluding that the arrangements were such as to favor self-fertilization, which is opposed to the generally accepted view.
Prof. L. M. Underwood, of Syracuse, N. Y., gave some statistics concerning the North-American Hepaticae. Of the two hundred and thirty-one species found north of Mexico, a hundred and twenty are pecular to America; fully one-half the latter are not represented in any public or private herbarium in this country.
In a paper on the nature of gumming, or gummosis, in fruit-trees, Prof. J. C. Arthur detailed experiments from which the conclusion had been reached that it was due to a deorganization of the cell-walls of the tree through the influence of some fungus, but not necessarily of a specific one.
It had been produced experimentally by the bacteria of pear-blight and by Monilia fructigenum, the fruit-rot fungus; although the most common cause is doubtless the Coryneum, first described by Oudemans in Hedwigia.
At the final meeting the Committee on Postal Matters then gave its report. This committee was appointed at Minneapolis to inquire into the various obstructions which the postal authorities throw in the way of exchanging specimens of dried plants. The efforts of the committee had been directed toward securing the passage of specimens bearing the customary written label at fourth-class rates of postage. The Decision of the Postmaster-General was read, stating that the present law could not be construed to permit the passage of specimens with written labels except at letter-rates, but expressing a willingness to bring the matter, at the proper time, 7462 to the attention of Congress, the Canadian authorities, and the congress of the Universal Postal Union. Some discussion followed; and a motion was carried to continue the committee, and also instructing the president and secretary of the club to draft resolutions to be presented to the section of biology, in order to still further promote the objects in view.
These resolutions were acted upon by the biological section on the following day. Dr. Bessey was chosen president, and Professor Arthur secretary, for the next year.
Besides the reading of papers, the club took several excursions. On Saturday they went to the pine-barrens of New Jersey, about fifty participating. On Monday a party visited the ballast-grounds during the morning, and upon their return inspected the library and herbarium of Mr. I. C. Martindale, of Camden, N. J. In the evening of the same day the Botanical section of the Philadelphia Academy of Science entertained the club, the Torrey Botanical Club of New-York City, and other invited guests, at the rooms of the Academy. About three hundred were present, and a thoroughly enjoyable time experienced. On the afternoon of Tuesday the club and its friends, in all about eighty, made an excursion to the Bartram Gardens, one of the most interesting historical spots to botanists in this country; and the club then adjourned.
In reviewing the attendance of botanists in Philadelphia, and the work of the Botanical Club, there is much reason for congratulation. About a hundred entered their names on the register of the club as botanists, or about eight per cent. of the total attendance, one-half of whom are widely known for their attainments in the science. There was no lack of interesting papers and free discussion. Besides the important measures already referred to, the club was instrumental in securing the appointment of a permanent committee of the Association to encourage researches on the health and diseases of plants. But, above all, the augmented facilities for intercourse and acquaintanceship, and the impulse imparted to individual workers, through the influence of the club, are a sufficient raison d'étre, and a promise of usefulness of the future.—Science.
The theory of artesian and of spouting petroleum wells is entirely different. While the latter owe their operation to an internal pressure, due to gases accumulated within a confined space, the former are due to the pressure of a liquid which is flowing—a pressure caused by a sheet of water of unequal height; and they spout with so much the more force in proportion as the difference of level between the orifice and starting point of the sheet of water is greater.
Petroleum reservoirs, or pockets, contain, along with the petroleum, gases, salt water, sand, and foreign substances of varying nature. The liquids and gases in these pockets are often submitted to very great pressure. If we make an aperture in the pocket, there will occur, by reason of the tension, and according to the location of the aperture, a sudden exit of gas, petroleum, salt water, etc. Yet it may happen that as the sounding well has been bored through the upper part of the pocket, where the gases are accumulated, only the latter will make their exit without any trace of petroleum. Under such circumstances the appearance of inflammable gases at the surface indicates pretty certainly the presence of inflammable liquids in the region explored, and will justify further exploration or the fitting of suction pumps to the well holes.
It will be understood that a natural flow of petroleum will occur only so long as the pressure is sufficient, and that a pocket may cease to give mineral oil spontaneously, even though it may still contain large quantities of it. This is the reason why at present spouting wells are not abandoned when they cease to operate, but are worked by lift pumps. The three diagrams, 1, 2, and 3, will give an idea of the different configurations that petroleum pockets may present. In No. 1, as the well hole reaches the summit of the gas chamber, the gases alone will be forced to the surface by reason of the internal pressure, and not the slightest trace of petroleum will accompany them.
In No. 2, as the well ends at the side of the pocket, only a portion of the petroleum—that which is included between the dotted lines—will come to the surface.
In No. 3, as the well ends at the lowest extremity of the pocket, nearly the entire contents of the latter will be forced out naturally. It results from this that in petroleum exploitation the sudden appearance and disappearance of the spouting in no wise proves that the pocket is exhausted.—Science et Nature.
Symbol, Al. Equivalent, old, 13.7; new. 27.49. Specific gravity, cast, 2.46. Hammered, 2.67. Specific heat, 0.2143, Heat conductivity, 0.66 on silver scale = 100.
Melting point, 1,250° or 1,560° Fah., according to different authorities.
A shining, white, sonorous metal, having a shade between silver and platinum. It is malleable and ductile, does not oxidize when exposed to dry or moist air, and is not chemically affected by hot or cold water.
Sulphureted hydrogen gas, which so readily tarnishes silver, has no action upon this metal.
Having but one defect in its uses as a pure metal (difficulty in soldering), it enters largely as an alloy of other metals, making the baser metals more valuable in resisting oxidation, and as a good as well as cheap imitation of the precious metals.
Its power to ameliorate the condition of the alloys of copper, zinc, tin, iron, nickel, silver, gold, and platinum by portions sometimes less than a thousandth part is beautifully illustrated in the elegant articles of tableware, bric a brac, and ornamental hardware now coming upon the commercial market. Its uses in the mechanic arts in the various forms of bronzes in filling a long wanted requirement of combined ductility, strength, sonorousness, and freedom from oxidation, thus giving to its alloys a high value for articles of house hardware, carriage and harness trimmings, quick running machinery, journal bearings, propeller blades, and artillery. Piano wires made from its alloys will vibrate ten seconds longer than the best now in use.
For the kitchen and for articles for the toilet, there is no more beautiful and cleanly ware. An alloy of silver 20 and aluminum 80 parts by weight, for nautical and other instruments, is without a rival in beauty and lightness; the sea air does not tarnish it.
The aluminum-silver alloys are more valuable than pure silver for table service; its wares will not be destroyed by the constant polishing that wears out our plate, and holds an immunity from the destructive effects of the fatty and acetic acids.
For watch cases it wears cleaner than pure silver, and for watch movements it is far superior to the brass and nickel or German silver heretofore used. An alloy is now made in France that has elastic qualities equal to steel for watch springs, and with the valuable property of being free from magnetic effect.
The aluminum bronzes, when combined with five per cent. of gold, have all the beauty, finish, and durability of color of eighteen carat gold; they are entering largely into the manufacture of watch cases and jewelry.
The composition most approved is made of copper 85, aluminum 10, gold 5, parts by weight. This can be soldered with any of the jeweler's solders of gold, silver, and zinc in the usual way.
The most important alloy, aluminum bronze, is composed of aluminum 10 parts, copper 90 parts by weight; specific gravity, 7.7. It has a pale gold color, harder than ordinary bronze, takes a fine polish, is malleable and ductile, but when rolled into sheets requires annealing at every third passage through the rolls, and when drawn into wire must be frequently annealed. It may be forged cold or hot, and can be drawn in tubes. In wire it has a tensile strength of 100,000 lb.
This alloy is often found to be brittle at the first mixing, but becomes ductile after remelting. It is softened while being worked by plunging in water at a low red heat.
The Parisian gold colored alloy is made of aluminum 10.7, copper, 89.3, by weight; used much for cheap French jewelry.
A non-oxidizable alloy in a moist atmosphere: Aluminum, 25, iron 75 = 25 per cent. aluminum. A hard bright alloy, with the properties of silver: Silver 5 (by weight); aluminum 95 = 5 per cent. aluminum.
The silver alloys with aluminum bronze, as represented in the four following atomic formulas, are of a rich gold color, and well adapted for jewelry, watch cases, etc.:
| Cu | Al | Ag | |
|---|---|---|---|
| Ag + 24 (Al + Cu6) = | 0.9180 | + 0.0616 | + 0.0203 |
| Ag + 24 (Al + Cu7) = | 0.9241 | + 0.0570 | + 0.0188 |
| Ag + 24 (Al + Cu8) = | 0.9330 | + 0.0504 | + 0.0166 |
| Ag + 24 (Al + Cu9) = | 0.9400 | + 0.0450 | + 0.0150 |
The figures being proportional weights.
A cheap alloy for journal boxes and machinery may be made by substituting zinc for silver in the following atomic proportions:
| Cu | Al | Zn | |
|---|---|---|---|
| Zn + 2(Al + Cu6) = | 0.8643 | + 0.0622 | + 0.0734 |
| Zn + 2(Al + Cu9) = | 0.9053 | + 0.0435 | + 0.0512 |
| Zn + 2(Al +Cu12) = | 0.9273 | + 0.0333 | + 0.0394 |
This is subject to considerable shrinkage in casting, but is tenacious, and when drawn into wire has a tensile strength of ninety to one hundred thousand pounds.
The following alloys, in which iron enters as a third element, are well adapted for gun metal, being hard, tenacious, laminable, and ductile:
| Cu | Al | Fe | |
|---|---|---|---|
| Fe + (Al + Cu15) = | 0.9203 | + 0.0267 | + 0.0530 |
| Fe + (Al + Cu9) = | 0.9399 | + 0.0446 | + 0.0149 |
Also a four-element alloy of
| Cu | Al | Zn | Fe | ||
|---|---|---|---|---|---|
| 1. | Fe + Zn + (Al + Cu12) = | 0.8386. | + 0.0305. | + 0.0712. | + 0.0600 |
| 2.. | Fe + Zn + (Al + Cu15) = | 0.8666. | + 0.0249. | + 0.0588. | + 0.0496 |
The tensile strength of the above alloys as drawn wire is 82,000 pounds for the first, and 107,000 pounds for the second.
All of the alloys in which zinc or zinc and iron enter in place of silver, the color is affected and the luster diminished.
With nickel and platinum for the third element, we have:
| Cu | Al | Ni | |
|---|---|---|---|
| Ni + 6 (Al + Cu6) = | 0.9129 | + 0.0634 | + 0.0237 |
| Pl + 21 (Al + Cu6) = | 0.9117 | + 0.0656 | + 0.0225 |
Those alloys into which platinum is introduced are less affected by acids than those in which silver takes the place of platinum; platinum producing a higher luster than silver.
In the alloys of aluminum bronze with the more difficultly fusible metals, it is preferable to fuse the bronze first, then add the other metal in small shavings or wire; by this means the less fusible metal absorbs the other without raising the heat of the furnace excessively. Add the least fusible metal last, a little at a time, allowing the heat of the melted metal to fall by degrees, which prevents boiling and evaporation. The crucibles for mixing the alloys should be of plumbago lined with a paste of lime.
Avoid sand crucibles, as silicium may be reduced and absorbed by the aluminum, inducing brittleness. If found brittle, remelt with cryolite as a flux, or stir the melted metal or alloy with a hard wood stick that has been slightly charred.
In adding aluminum to the copper, cut it in small pieces and push it to the bottom of the crucible with a dry, hard wood stick split so as to hold the pieces.
Sodium chloride (common salt) calcined to evaporate the water, and caustic soda with pulverized charcoal, may be used as a flux for pure aluminum. Avoid borax as a flux, as its metal may suffer reduction, making the aluminum brittle. Aluminum will alloy with tin alone, but is liable to separate on refusion. Does not alloy with lead.
Bismuth, even in minute quantity, makes these alloys brittle.
The East Indian steel called wootz is, according to analysis, alloyed with aluminum. No reliable solder has yet been found for pure aluminum that will flow freely under the blow pipe or from a soldering iron.
A process recently adopted in France is to plate the parts to be united with alloys of tin 5, aluminum 1, upon which tin solder will flow. These proportions may be slightly varied to suit requirements for hardness.
Harder solders to be used with a blowpipe may be made with alloys of zinc, tin, and aluminum.
Aluminum is now made at the works of M. Deville, at Javelle, near Paris, and at Salindres, France; also at Birmingham, England. The product of late has reached the value of $20,000 annually in Europe. It has been claimed to be made in Philadelphia at a reduced cost. The present price in New York is $1.25 per oz. As its bulk is over four times as great as silver, its comparative cost is but one-third that of silver—a point not often considered when the price is quoted.
A CATALOGUE containing brief notices of many important scientific papers heretofore published in the Supplement, may be had gratis at this office.
Sent by mail, postage prepaid, to subscribers in any part of the United States or Canada. Six dollars a year, sent, prepaid, to any foreign country.
All the back numbers of The Supplement, from the commencement, January 1, 1876, can be had. Price, 10 cents each.
All the back volumes of The Supplement can likewise be supplied. Two volumes are issued yearly. Price of each volume, $2.50, stitched in paper, or $3.50, bound in stiff covers.
Combined Rates—One copy of Scientific American and one copy of Scientific American Supplement, one year, postpaid, $7.00.
A liberal discount to booksellers, news agents, and canvassers.
MUNN & CO., Publishers,
361 Broadway, New York, N. Y.
In connection with the Scientific American, Messrs. Munn & Co. are Solicitors of American and Foreign Patents, have had 39 years' experience, and now have the largest establishment in the world. Patents are obtained on the best terms.
A special notice is made in the Scientific American of all Inventions patented through this Agency, with the name and residence of the Patentee. By the immense circulation thus given, public attention is directed to the merits of the new patent, and sales or introduction often easily effected.
Any person who has made a new discovery or invention can ascertain, free of charge, whether a patent can probably be obtained, by writing to Munn & Co.
We also send free our Hand Book about the Patent Laws, Patents, Caveats, Trade Marks, their costs, and how procured. Address
MUNN & CO.. 361 Broadway, New York.
Branch Office, cor. F and 7th Sts., Washington, D. C.
1 A Lecture delivered at the Academy of Music, Philadelphia, under the auspices of the Franklin Institute, September 29, 1884.
2 Alluding to a moving diagram of wave motion of sound produced by a working slide for lantern projection.
3 Showing two moving diagrams, simultaneously, on the screen, depicting a wave motion of light, the other a sound vibration.
4 Exhibiting a large drawing, or chart, representing a red and a violet wave of light.
5 Since my lecture I have heard from Prof. Langley that he has measured the refrangibility by a rock salt prism, and inferred the wave length of heat rays from a "Leslie cube" (a metal vessel of hot water radiating from a blackened side). The greatest wave length he has thus found is one one-thousandth of a centimeter, which is seventeen times that of sodium light. The corresponding period is about thirty million million to the second.—W.T.
6 Exhibiting a large bowl of clear jelly with a small red wooden ball embedded in the surface near the center.
7 Showing the chromatic bands thrown upon the screen from a diffraction grating.
8 Reproduced in abridged form from the Electrical Review and the cuts from La Lumiere Electrique.—Science.
9 See Supplement No. 264 for an illustrated description.
10 Annales Industrielles.
11 A communication to the London and Provincial Photographic Association.
12 Translated from the Revue Odontologique, for the Dental Cosmos.
13 In the cuts, Nos. 6, 7, and 8 are proportionate modifications of No. 5.
14 By Professor Henry Robinson. Paper read Oct. 2, 1884, at the Congress of the Institute held at Dublin.—Building News.
Transcriber's Note:
Inconsistent spelling and hyphenation are as in the original.
End of the Project Gutenberg EBook of Scientific American Supplement, No.
467, December 13, 1884, by Various
*** END OF THIS PROJECT GUTENBERG EBOOK SCIENTIFIC AMERICAN SUPP., DEC 13, 1884 ***
***** This file should be named 46706-h.htm or 46706-h.zip *****
This and all associated files of various formats will be found in:
http://www.gutenberg.org/4/6/7/0/46706/
Produced by Juliet and other, Juliet Sutherland, Wayne
Hammond and the Online Distributed Proofreading Team at
http://www.pgdp.net
Updated editions will replace the previous one--the old editions will
be renamed.
Creating the works from print editions not protected by U.S. copyright
law means that no one owns a United States copyright in these works,
so the Foundation (and you!) can copy and distribute it in the United
States without permission and without paying copyright
royalties. Special rules, set forth in the General Terms of Use part
of this license, apply to copying and distributing Project
Gutenberg-tm electronic works to protect the PROJECT GUTENBERG-tm
concept and trademark. Project Gutenberg is a registered trademark,
and may not be used if you charge for the eBooks, unless you receive
specific permission. If you do not charge anything for copies of this
eBook, complying with the rules is very easy. You may use this eBook
for nearly any purpose such as creation of derivative works, reports,
performances and research. They may be modified and printed and given
away--you may do practically ANYTHING in the United States with eBooks
not protected by U.S. copyright law. Redistribution is subject to the
trademark license, especially commercial redistribution.
START: FULL LICENSE
THE FULL PROJECT GUTENBERG LICENSE
PLEASE READ THIS BEFORE YOU DISTRIBUTE OR USE THIS WORK
To protect the Project Gutenberg-tm mission of promoting the free
distribution of electronic works, by using or distributing this work
(or any other work associated in any way with the phrase "Project
Gutenberg"), you agree to comply with all the terms of the Full
Project Gutenberg-tm License available with this file or online at
www.gutenberg.org/license.
Section 1. General Terms of Use and Redistributing Project
Gutenberg-tm electronic works
1.A. By reading or using any part of this Project Gutenberg-tm
electronic work, you indicate that you have read, understand, agree to
and accept all the terms of this license and intellectual property
(trademark/copyright) agreement. If you do not agree to abide by all
the terms of this agreement, you must cease using and return or
destroy all copies of Project Gutenberg-tm electronic works in your
possession. If you paid a fee for obtaining a copy of or access to a
Project Gutenberg-tm electronic work and you do not agree to be bound
by the terms of this agreement, you may obtain a refund from the
person or entity to whom you paid the fee as set forth in paragraph
1.E.8.
1.B. "Project Gutenberg" is a registered trademark. It may only be
used on or associated in any way with an electronic work by people who
agree to be bound by the terms of this agreement. There are a few
things that you can do with most Project Gutenberg-tm electronic works
even without complying with the full terms of this agreement. See
paragraph 1.C below. There are a lot of things you can do with Project
Gutenberg-tm electronic works if you follow the terms of this
agreement and help preserve free future access to Project Gutenberg-tm
electronic works. See paragraph 1.E below.
1.C. The Project Gutenberg Literary Archive Foundation ("the
Foundation" or PGLAF), owns a compilation copyright in the collection
of Project Gutenberg-tm electronic works. Nearly all the individual
works in the collection are in the public domain in the United
States. If an individual work is unprotected by copyright law in the
United States and you are located in the United States, we do not
claim a right to prevent you from copying, distributing, performing,
displaying or creating derivative works based on the work as long as
all references to Project Gutenberg are removed. Of course, we hope
that you will support the Project Gutenberg-tm mission of promoting
free access to electronic works by freely sharing Project Gutenberg-tm
works in compliance with the terms of this agreement for keeping the
Project Gutenberg-tm name associated with the work. You can easily
comply with the terms of this agreement by keeping this work in the
same format with its attached full Project Gutenberg-tm License when
you share it without charge with others.
1.D. The copyright laws of the place where you are located also govern
what you can do with this work. Copyright laws in most countries are
in a constant state of change. If you are outside the United States,
check the laws of your country in addition to the terms of this
agreement before downloading, copying, displaying, performing,
distributing or creating derivative works based on this work or any
other Project Gutenberg-tm work. The Foundation makes no
representations concerning the copyright status of any work in any
country outside the United States.
1.E. Unless you have removed all references to Project Gutenberg:
1.E.1. The following sentence, with active links to, or other
immediate access to, the full Project Gutenberg-tm License must appear
prominently whenever any copy of a Project Gutenberg-tm work (any work
on which the phrase "Project Gutenberg" appears, or with which the
phrase "Project Gutenberg" is associated) is accessed, displayed,
performed, viewed, copied or distributed:
This eBook is for the use of anyone anywhere in the United States and
most other parts of the world at no cost and with almost no
restrictions whatsoever. You may copy it, give it away or re-use it
under the terms of the Project Gutenberg License included with this
eBook or online at www.gutenberg.org. If you are not located in the
United States, you'll have to check the laws of the country where you
are located before using this ebook.
1.E.2. If an individual Project Gutenberg-tm electronic work is
derived from texts not protected by U.S. copyright law (does not
contain a notice indicating that it is posted with permission of the
copyright holder), the work can be copied and distributed to anyone in
the United States without paying any fees or charges. If you are
redistributing or providing access to a work with the phrase "Project
Gutenberg" associated with or appearing on the work, you must comply
either with the requirements of paragraphs 1.E.1 through 1.E.7 or
obtain permission for the use of the work and the Project Gutenberg-tm
trademark as set forth in paragraphs 1.E.8 or 1.E.9.
1.E.3. If an individual Project Gutenberg-tm electronic work is posted
with the permission of the copyright holder, your use and distribution
must comply with both paragraphs 1.E.1 through 1.E.7 and any
additional terms imposed by the copyright holder. Additional terms
will be linked to the Project Gutenberg-tm License for all works
posted with the permission of the copyright holder found at the
beginning of this work.
1.E.4. Do not unlink or detach or remove the full Project Gutenberg-tm
License terms from this work, or any files containing a part of this
work or any other work associated with Project Gutenberg-tm.
1.E.5. Do not copy, display, perform, distribute or redistribute this
electronic work, or any part of this electronic work, without
prominently displaying the sentence set forth in paragraph 1.E.1 with
active links or immediate access to the full terms of the Project
Gutenberg-tm License.
1.E.6. You may convert to and distribute this work in any binary,
compressed, marked up, nonproprietary or proprietary form, including
any word processing or hypertext form. However, if you provide access
to or distribute copies of a Project Gutenberg-tm work in a format
other than "Plain Vanilla ASCII" or other format used in the official
version posted on the official Project Gutenberg-tm web site
(www.gutenberg.org), you must, at no additional cost, fee or expense
to the user, provide a copy, a means of exporting a copy, or a means
of obtaining a copy upon request, of the work in its original "Plain
Vanilla ASCII" or other form. Any alternate format must include the
full Project Gutenberg-tm License as specified in paragraph 1.E.1.
1.E.7. Do not charge a fee for access to, viewing, displaying,
performing, copying or distributing any Project Gutenberg-tm works
unless you comply with paragraph 1.E.8 or 1.E.9.
1.E.8. You may charge a reasonable fee for copies of or providing
access to or distributing Project Gutenberg-tm electronic works
provided that
* You pay a royalty fee of 20% of the gross profits you derive from
the use of Project Gutenberg-tm works calculated using the method
you already use to calculate your applicable taxes. The fee is owed
to the owner of the Project Gutenberg-tm trademark, but he has
agreed to donate royalties under this paragraph to the Project
Gutenberg Literary Archive Foundation. Royalty payments must be paid
within 60 days following each date on which you prepare (or are
legally required to prepare) your periodic tax returns. Royalty
payments should be clearly marked as such and sent to the Project
Gutenberg Literary Archive Foundation at the address specified in
Section 4, "Information about donations to the Project Gutenberg
Literary Archive Foundation."
* You provide a full refund of any money paid by a user who notifies
you in writing (or by e-mail) within 30 days of receipt that s/he
does not agree to the terms of the full Project Gutenberg-tm
License. You must require such a user to return or destroy all
copies of the works possessed in a physical medium and discontinue
all use of and all access to other copies of Project Gutenberg-tm
works.
* You provide, in accordance with paragraph 1.F.3, a full refund of
any money paid for a work or a replacement copy, if a defect in the
electronic work is discovered and reported to you within 90 days of
receipt of the work.
* You comply with all other terms of this agreement for free
distribution of Project Gutenberg-tm works.
1.E.9. If you wish to charge a fee or distribute a Project
Gutenberg-tm electronic work or group of works on different terms than
are set forth in this agreement, you must obtain permission in writing
from both the Project Gutenberg Literary Archive Foundation and The
Project Gutenberg Trademark LLC, the owner of the Project Gutenberg-tm
trademark. Contact the Foundation as set forth in Section 3 below.
1.F.
1.F.1. Project Gutenberg volunteers and employees expend considerable
effort to identify, do copyright research on, transcribe and proofread
works not protected by U.S. copyright law in creating the Project
Gutenberg-tm collection. Despite these efforts, Project Gutenberg-tm
electronic works, and the medium on which they may be stored, may
contain "Defects," such as, but not limited to, incomplete, inaccurate
or corrupt data, transcription errors, a copyright or other
intellectual property infringement, a defective or damaged disk or
other medium, a computer virus, or computer codes that damage or
cannot be read by your equipment.
1.F.2. LIMITED WARRANTY, DISCLAIMER OF DAMAGES - Except for the "Right
of Replacement or Refund" described in paragraph 1.F.3, the Project
Gutenberg Literary Archive Foundation, the owner of the Project
Gutenberg-tm trademark, and any other party distributing a Project
Gutenberg-tm electronic work under this agreement, disclaim all
liability to you for damages, costs and expenses, including legal
fees. YOU AGREE THAT YOU HAVE NO REMEDIES FOR NEGLIGENCE, STRICT
LIABILITY, BREACH OF WARRANTY OR BREACH OF CONTRACT EXCEPT THOSE
PROVIDED IN PARAGRAPH 1.F.3. YOU AGREE THAT THE FOUNDATION, THE
TRADEMARK OWNER, AND ANY DISTRIBUTOR UNDER THIS AGREEMENT WILL NOT BE
LIABLE TO YOU FOR ACTUAL, DIRECT, INDIRECT, CONSEQUENTIAL, PUNITIVE OR
INCIDENTAL DAMAGES EVEN IF YOU GIVE NOTICE OF THE POSSIBILITY OF SUCH
DAMAGE.
1.F.3. LIMITED RIGHT OF REPLACEMENT OR REFUND - If you discover a
defect in this electronic work within 90 days of receiving it, you can
receive a refund of the money (if any) you paid for it by sending a
written explanation to the person you received the work from. If you
received the work on a physical medium, you must return the medium
with your written explanation. The person or entity that provided you
with the defective work may elect to provide a replacement copy in
lieu of a refund. If you received the work electronically, the person
or entity providing it to you may choose to give you a second
opportunity to receive the work electronically in lieu of a refund. If
the second copy is also defective, you may demand a refund in writing
without further opportunities to fix the problem.
1.F.4. Except for the limited right of replacement or refund set forth
in paragraph 1.F.3, this work is provided to you 'AS-IS', WITH NO
OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING BUT NOT
LIMITED TO WARRANTIES OF MERCHANTABILITY OR FITNESS FOR ANY PURPOSE.
1.F.5. Some states do not allow disclaimers of certain implied
warranties or the exclusion or limitation of certain types of
damages. If any disclaimer or limitation set forth in this agreement
violates the law of the state applicable to this agreement, the
agreement shall be interpreted to make the maximum disclaimer or
limitation permitted by the applicable state law. The invalidity or
unenforceability of any provision of this agreement shall not void the
remaining provisions.
1.F.6. INDEMNITY - You agree to indemnify and hold the Foundation, the
trademark owner, any agent or employee of the Foundation, anyone
providing copies of Project Gutenberg-tm electronic works in
accordance with this agreement, and any volunteers associated with the
production, promotion and distribution of Project Gutenberg-tm
electronic works, harmless from all liability, costs and expenses,
including legal fees, that arise directly or indirectly from any of
the following which you do or cause to occur: (a) distribution of this
or any Project Gutenberg-tm work, (b) alteration, modification, or
additions or deletions to any Project Gutenberg-tm work, and (c) any
Defect you cause.
Section 2. Information about the Mission of Project Gutenberg-tm
Project Gutenberg-tm is synonymous with the free distribution of
electronic works in formats readable by the widest variety of
computers including obsolete, old, middle-aged and new computers. It
exists because of the efforts of hundreds of volunteers and donations
from people in all walks of life.
Volunteers and financial support to provide volunteers with the
assistance they need are critical to reaching Project Gutenberg-tm's
goals and ensuring that the Project Gutenberg-tm collection will
remain freely available for generations to come. In 2001, the Project
Gutenberg Literary Archive Foundation was created to provide a secure
and permanent future for Project Gutenberg-tm and future
generations. To learn more about the Project Gutenberg Literary
Archive Foundation and how your efforts and donations can help, see
Sections 3 and 4 and the Foundation information page at
www.gutenberg.org Section 3. Information about the Project Gutenberg
Literary Archive Foundation
The Project Gutenberg Literary Archive Foundation is a non profit
501(c)(3) educational corporation organized under the laws of the
state of Mississippi and granted tax exempt status by the Internal
Revenue Service. The Foundation's EIN or federal tax identification
number is 64-6221541. Contributions to the Project Gutenberg Literary
Archive Foundation are tax deductible to the full extent permitted by
U.S. federal laws and your state's laws.
The Foundation's principal office is in Fairbanks, Alaska, with the
mailing address: PO Box 750175, Fairbanks, AK 99775, but its
volunteers and employees are scattered throughout numerous
locations. Its business office is located at 809 North 1500 West, Salt
Lake City, UT 84116, (801) 596-1887. Email contact links and up to
date contact information can be found at the Foundation's web site and
official page at www.gutenberg.org/contact
For additional contact information:
Dr. Gregory B. Newby
Chief Executive and Director
gbnewby@pglaf.org
Section 4. Information about Donations to the Project Gutenberg
Literary Archive Foundation
Project Gutenberg-tm depends upon and cannot survive without wide
spread public support and donations to carry out its mission of
increasing the number of public domain and licensed works that can be
freely distributed in machine readable form accessible by the widest
array of equipment including outdated equipment. Many small donations
($1 to $5,000) are particularly important to maintaining tax exempt
status with the IRS.
The Foundation is committed to complying with the laws regulating
charities and charitable donations in all 50 states of the United
States. Compliance requirements are not uniform and it takes a
considerable effort, much paperwork and many fees to meet and keep up
with these requirements. We do not solicit donations in locations
where we have not received written confirmation of compliance. To SEND
DONATIONS or determine the status of compliance for any particular
state visit www.gutenberg.org/donate
While we cannot and do not solicit contributions from states where we
have not met the solicitation requirements, we know of no prohibition
against accepting unsolicited donations from donors in such states who
approach us with offers to donate.
International donations are gratefully accepted, but we cannot make
any statements concerning tax treatment of donations received from
outside the United States. U.S. laws alone swamp our small staff.
Please check the Project Gutenberg Web pages for current donation
methods and addresses. Donations are accepted in a number of other
ways including checks, online payments and credit card donations. To
donate, please visit: www.gutenberg.org/donate
Section 5. General Information About Project Gutenberg-tm electronic works.
Professor Michael S. Hart was the originator of the Project
Gutenberg-tm concept of a library of electronic works that could be
freely shared with anyone. For forty years, he produced and
distributed Project Gutenberg-tm eBooks with only a loose network of
volunteer support.
Project Gutenberg-tm eBooks are often created from several printed
editions, all of which are confirmed as not protected by copyright in
the U.S. unless a copyright notice is included. Thus, we do not
necessarily keep eBooks in compliance with any particular paper
edition.
Most people start at our Web site which has the main PG search
facility: www.gutenberg.org
This Web site includes information about Project Gutenberg-tm,
including how to make donations to the Project Gutenberg Literary
Archive Foundation, how to help produce our new eBooks, and how to
subscribe to our email newsletter to hear about new eBooks.